In brief
MIT combustion experts have designed a system that uses flames to produce materials for cathodes of lithium-ion batteries—materials that now contribute to both the high cost and the high performance of those batteries. Based on extensive lab-scale experiments, the researchers’ system promises to be simpler, much quicker, and far less energy-intensive than the conventional method now used to manufacture cathode materials. Electrochemical tests show that their materials should produce lithium-ion batteries that perform as well as those used in electric vehicles today, providing a comparable driving range, charge and discharge rate, and lifetime. The components in the system are already used in industry, so the researchers believe that rapid commercialization and scale-up should be possible.
For more than a century, much of the world has run on the combustion of fossil fuels. Now, to avert the threat of climate change, the energy system is changing. Notably, solar and wind systems are replacing fossil fuel combustion for generating electricity and heat, and batteries are replacing the internal combustion engine for powering vehicles. As the energy transition progresses, researchers worldwide are tackling the many challenges that arise.
Sili Deng has spent her career thinking about combustion. Now an assistant professor in the Department of Mechanical Engineering and the Class of 1954 Career Development Professor, Deng leads an MIT group that, among other things, develops theoretical models to help understand and control combustion systems to make them more efficient and to control the formation of emissions, including particles of soot.
“So we thought, given our background in combustion, what’s the best way we can contribute to the energy transition?” says Deng. In considering the possibilities, she notes that combustion refers only to the process—not to what’s burning. “While we generally think of fossil fuels when we think of combustion, the term ‘combustion’ encompasses many high-temperature chemical reactions that involve oxygen and typically emit light and large amounts of heat,” she says.
Given that definition, she saw another role for the expertise she and her team have developed: They could explore the use of combustion to make materials for the energy transition. Under carefully controlled conditions, combusting flames can be used to produce not polluting soot but rather valuable materials, including some that are critical in the manufacture of lithium-ion batteries.
Improving the lithium-ion battery by lowering costs
The demand for lithium-ion batteries is projected to skyrocket in the coming decades. Batteries will be needed to power the growing fleet of electric cars and to store the electricity produced by solar and wind systems so it can be delivered later when those sources aren’t generating. Some experts project that the global demand for lithium-ion batteries may increase tenfold or more in the next decade.
Given such projections, many researchers are looking for ways to improve the lithium-ion battery technology. Deng and her group aren’t materials scientists, so they don’t focus on making new and better battery chemistries. Instead, their goal is to find a way to lower the high cost of making all of those batteries. And much of the cost of making a lithium-ion battery can be traced to the manufacture of materials used to make one of its two electrodes—the cathode.
The MIT researchers began their search for cost savings by considering the methods now used to produce cathode materials. The raw materials are typically salts of several metals, including lithium, which provides ions—the electrically charged particles that move when the battery is charged and discharged. The processing technology aims to produce tiny particles, each one made up of a mixture of those ingredients with the atoms arranged in the specific crystalline structure that will deliver the best performance in the finished battery.
For the past several decades, companies have manufactured those cathode materials using a two-stage process called coprecipitation. In the first stage, the metal salts—excluding the lithium—are dissolved in water and thoroughly mixed inside a chemical reactor. Chemicals are added to change the acidity (the pH) of the mixture, and particles made up of the combined salts precipitate out of the solution. The particles are then removed, dried, ground up, and put through a sieve.
A change in pH won’t cause lithium to precipitate, so it is added in the second stage. Solid lithium is ground together with the particles from the first stage until lithium atoms permeate the particles. The resulting material is then heated, or “annealed,” to ensure complete mixing and to achieve the targeted crystalline structure. Finally, the particles go through a “deagglomerator” that separates any particles that have joined together, and the cathode material emerges.
Coprecipitation produces the needed materials, but the process is time-consuming. The first stage takes about 10 hours, and the second stage requires about 13 hours of annealing at a relatively low temperature (750°C). In addition, to prevent cracking during annealing, the temperature is gradually “ramped” up and down, which takes another 11 hours. The process is thus not only time-consuming but also energy-intensive and costly.
For the past two years, Deng and her group have been exploring better ways to make the cathode material. “Combustion is very effective at oxidizing things, and the materials for lithium-ion batteries are generally mixtures of metal oxides,” says Deng. That being the case, they thought this could be an opportunity to use a combustion-based process called flame synthesis.
A new way of making a high-performance cathode material
The first task for Deng and her team—mechanical engineering postdoc Jianan Zhang, Valerie L. Muldoon SB ’20, SM ’22, and current graduate students Maanasa Bhat and Chuwei Zhang—was to choose a target material for their study. They decided to focus on a mixture of metal oxides consisting of nickel, cobalt, and manganese plus lithium. Known as “NCM811,” this material is widely used and has been shown to produce cathodes for batteries that deliver high performance; in an electric vehicle, that means a long driving range, rapid discharge and recharge, and a long lifetime. To better define their target, the researchers examined the literature to determine the composition and crystalline structure of NCM811 that has been shown to deliver the best performance as a cathode material.
They then considered three possible approaches to improving on the coprecipitation process for synthesizing NCM811: They could simplify the system (to cut capital costs), speed up the process, or cut the energy required.
“Our first thought was, what if we can mix together all of the substances—including the lithium—at the beginning?” says Deng. “Then we would not need to have the two stages”—a clear simplification over coprecipitation.
Introducing FASP
One process widely used in the chemical and other industries to fabricate nanoparticles is a type of flame synthesis called flame-assisted spray pyrolysis, or FASP. Deng’s concept for using FASP to make their targeted cathode powders appears in the schematic below.
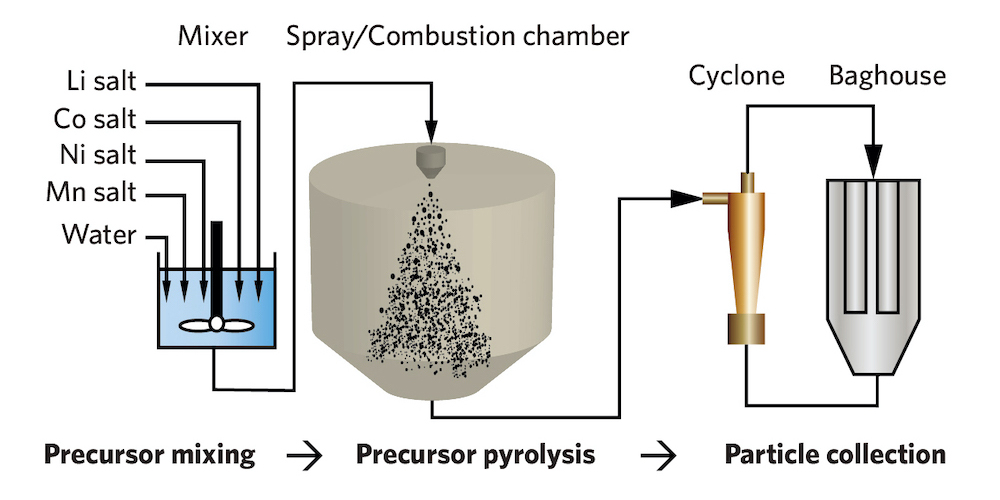
Synthesizing cathode materials using flame-assisted spray pyrolysis (FASP). From the left: In the researchers’ system, lithium, cobalt, nickel, and manganese salts are mixed with water and sprayed as fine droplets into a combustion chamber. Inside that chamber, a flame of burning methane heats the salts, causing them to decompose and chemically react—a process called pyrolysis. The water evaporates, and the solid particles left behind are sorted and filtered by size in the cyclone and the baghouse to produce the cathode powder. The powders could then undergo the standard annealing process, but the researchers have found a faster, more energy-efficient way to accomplish that final step.
At the left, the precursor materials—the metal salts (including the lithium)—are mixed with water, and the resulting solution is sprayed as fine droplets by an atomizer into a combustion chamber. There, a flame of burning methane heats up the mixture. The water evaporates, leaving the precursor materials to decompose, oxidize, and solidify to form the powder product. The cyclone separates particles of different sizes, and the baghouse filters out those that aren’t useful. The collected particles would then be annealed and deagglomerated.
To investigate and optimize this concept, the researchers developed a lab-scale FASP setup consisting of a homemade ultrasonic nebulizer, a preheating section, a burner, a filter, and a vacuum pump that withdraws the powders that form. Using that system, they could control the details of the heating process: The preheating section replicates conditions as the material first enters the combustion chamber, and the burner replicates conditions as it passes the flame. That setup allowed the team to explore operating conditions that would give the best results.
Their experiments showed marked benefits over coprecipitation. The nebulizer breaks up the liquid solution into fine droplets, ensuring atomic-level mixing. The water simply evaporates, so there’s no need to change the pH or to separate the solids from a liquid. As Deng notes, “You just let the gas go, and you’re left with the particles, which is what you want.” With lithium included at the outset, there’s no need for mixing solids with solids, which is neither efficient nor effective.

Postdoc Jianan Zhang heats and compresses powders produced in the lab-scale FASP setup prior to fabricating a cell for performance tests. Credit: Gretchen Ertl
They could even control the structure, or “morphology,” of the particles that formed. In one series of experiments, they tried exposing the incoming spray to different rates of temperature change over time. They found that the temperature “history” has a direct impact on morphology. With no preheating, the particles burst apart; and with rapid preheating, the particles were hollow. The best outcomes came when they used temperatures ranging from 175°C to 225°C. Experiments with coin-cell batteries (laboratory devices used for testing battery materials) confirmed that by adjusting the preheating temperature, they could achieve a particle morphology that would optimize the performance of their materials.
Best of all, the particles formed in seconds. Assuming the time needed for conventional annealing and deagglomerating, the new setup could synthesize the finished cathode material in half the total time needed for coprecipitation. Moreover, the first stage of the coprecipitation system is replaced by a far simpler setup—a savings in capital costs.
“We were very happy,” says Deng. “But then we thought, if we’ve changed the precursor side so the lithium is mixed well with the salts, do we need to have the same process for the second stage? Maybe not!”

A container of coin-cell batteries ready for testing. Credit: Gretchen Ertl
Improving the second stage
The key time- and energy-consuming step in the second stage is the annealing. In today’s coprecipitation process, the strategy is to anneal at a low temperature for a long time, giving the operator time to manipulate and control the process. But running a furnace for some 20 hours—even at a low temperature—consumes a lot of energy.
Based on their studies thus far, Deng thought, “What if we slightly increase the temperature but reduce the annealing time by orders of magnitude? Then we could cut energy consumption, and we might still achieve the desired crystal structure.”
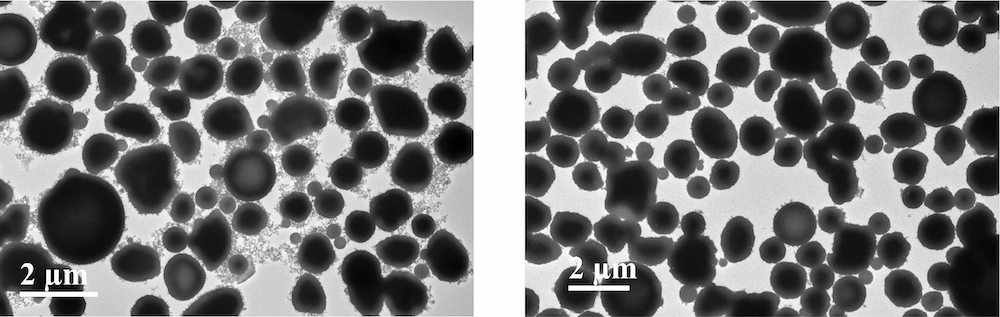
Elevating the temperature and shortening the duration of annealing. Initial experiments treating the as-synthesized powders at slightly elevated temperatures and short treatment times produced the particles shown in the transmission electron microscope (TEM) image on the left. The light nanoscale particles that appear in clouds on their surfaces are lithium, suggesting that far more time is needed to get the lithium to mix in. Performing the same experiments after adding a small amount of an inexpensive compound called urea produced the particles in the TEM image on the right, which show few attached lithium nanoparticles.
However, experiments at slightly elevated temperatures and short treatment times didn’t bring the results they hoped for. As the left-hand transmission electron microscope (TEM) image above shows, the particles that formed had clouds of light-looking nanoscale particles attached to their surfaces. When they performed the same experiments without adding the lithium, those nanoparticles didn’t appear. Based on that and other tests, they concluded that the nanoparticles were pure lithium. So, it seemed like long-duration annealing would be needed to ensure that the lithium made its way inside the particles.
But they then came up with a different solution to the lithium-distribution problem. They added a small amount—just 1% by weight—of an inexpensive compound called urea to their mixture. As the right-hand TEM image above shows, the “undesirable nanoparticles were then largely gone,” says Deng.
Experiments in the laboratory coin cells showed that the addition of urea significantly altered the response to changes in the annealing temperature. When the urea was absent, raising the annealing temperature led to a dramatic decline in performance of the cathode material that formed. But with the urea present, the performance of the material that formed was unaffected by any temperature change.

Using a sample of their powders, graduate student Chuwei Zhang (left) assembles a coin-cell battery for performance testing, while Assistant Professor Sili Deng looks on. Working inside a “glove box” is critical because the electrolyte is flammable and the electrodes can degrade in air. Credit: Gretchen Ertl
That result meant that—as long as the urea was added with the other precursors—they could push up the temperature, shrink the annealing time, and omit the gradual ramp-up and cool-down process. Further imaging studies confirmed that their approach yields the desired crystal structure and the homogeneous elemental distribution of the cobalt, nickel, manganese, and lithium within the particles. Moreover, in tests of various performance measures, their materials did as well as materials produced by coprecipitation or by other methods using long-time heat treatment. Indeed, the performance was comparable to that of commercial batteries with cathodes made of NCM811.
So now the long and expensive second stage required in standard coprecipitation could be replaced by just 20 minutes of annealing at about 870°C plus 20 minutes of cooling down at room temperature.

The team discusses results of performance tests of coin-cell batteries made using powders synthesized in the lab-scale FASP apparatus under carefully controlled conditions. During testing, the batteries are placed into a series of clamps, and measurements of flowing current and remaining capacity over time are displayed for each one on the computer monitor at the lower right. Credit: Gretchen Ertl
Theory, continuing work, and planning for scale-up
While experimental evidence supports their approach, Deng and her group are now working to understand why it works. “Getting the underlying physics right will help us design the process to control the morphology and to scale up the process,” says Deng.
The figure below presents their hypothesis for why the lithium nanoparticles in their flame synthesis process end up on the surfaces of the larger particles—and why the presence of urea solves that problem.
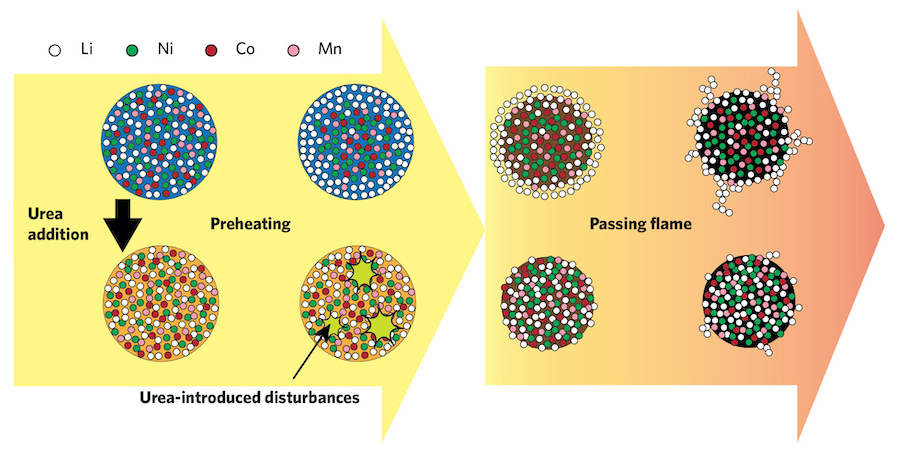
The fate of the lithium, without and with the urea additive: A proposed mechanism. The researchers theorize that without the urea present—the top row of drawings—all the metal salts start out well-mixed within the droplet; but over time, the lithium diffuses to the surface. The lithium ends up in nanoparticles attached to the solidified particle, and a long heat treatment is needed for the lithium to mix in. When the urea is present—the bottom row of drawings—the rising temperature causes the urea to form bubbles. The bubbles burst, increasing circulation, which keeps the lithium from diffusing to the surface. The lithium is distributed uniformly, so the final heat treatment can be very short.
The top series of drawings shows a droplet made of lithium and the other metal salts, without any urea present. In the sketch at the left, the metal and lithium atoms are well-mixed. Moving to the right, the lithium nanoparticles rise toward the surface; then they appear in a surface coating; and—after the droplet is heated by the flame and becomes a solid—they end up as nanoparticles loosely attached to the particle surface. The researchers’ theory? As the droplets evolve, atoms of the different metals remain mixed, but the lithium diffuses rapidly to the surface and remains there as the particle solidifies. Therefore, a long annealing process is needed to move the lithium in among the other atoms.
With the urea present, the lithium mixes in. Why? The bottom row of sketches shows their theory. As the first sketch shows, the urea and the lithium both mix with the other atoms. When the urea is heated, it decomposes, forming bubbles that pop. That popping enhances mixing inside the droplet, so the lithium doesn’t rise to the surface but instead remains mixed up with the rest of the atoms. Because the lithium is already mostly mixed throughout, the annealing time that follows can be very short.
The researchers are now designing a system to suspend a droplet of their mixture so they can observe the circulation inside it, with and without the urea present. They’re also developing experiments to examine how droplets vaporize, employing tools and methods they have used in the past to study how hydrocarbons vaporize inside internal combustion engines.
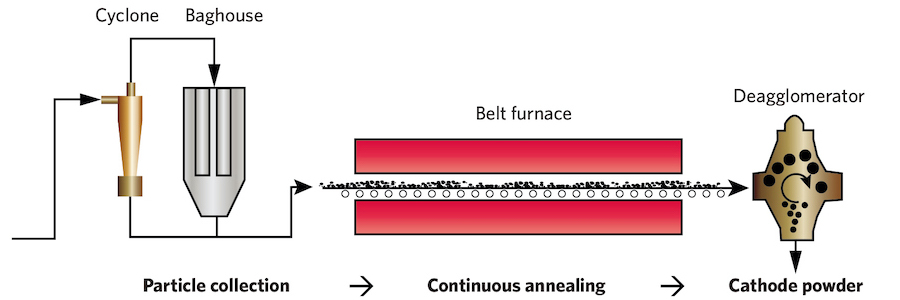
Design of an integrated system for manufacturing cathode materials. The FASP system previously illustrated will produce particles in 20 minutes or less. At that rate, they can be continuously deposited from the baghouse onto a belt that carries them through a furnace for just 10 to 20 minutes, as shown in this diagram above. A deagglomerator is still needed to break apart any particles that have become attached. The cathode powders for high-performance lithium-ion batteries could thus be manufactured at unprecedented speed, low cost, and low energy use. The components in the complete FASP-based system are already used in industry, so the researchers believe that rapid commercialization and scale-up should be possible.
They also have ideas about how to streamline and scale up their process. One concept is illustrated above. Their novel FASP process generates particles in 20 minutes or less—a rate that’s consistent with continuous processing. In coprecipitation, the first stage takes 10 to 20 hours, so one batch at a time moves on to the second stage to be annealed. With FASP, the particles coming out of the baghouse are deposited on a belt that carries them for 10 or 20 minutes through a furnace, as shown in the illustration. A deagglomerator then breaks any attached particles apart, and the cathode powder comes out, ready to be fabricated into a high-performance cathode for a lithium-ion battery.
Deng notes that every component in their “integrated synthesis system” is already used in industry, generally at a large scale and high flow-through rate. “That’s why we see great potential for our technology to be commercialized and scaled up,” she says. “Where our expertise comes into play is in designing the combustion chamber to control the temperature and heating rate so as to produce particles with the desired morphology.” And while a detailed economic analysis has yet to be performed, it seems clear that their technique will be faster, the equipment simpler, and the energy use lower than other methods of manufacturing cathode materials for lithium-ion batteries—potentially a major contribution to the ongoing energy transition.

Graduate student Maanasa Bhat has developed a device in which she can observe the circulation within an individual droplet as it evaporates. Developing a fundamental understanding of the physics controlling the synthesis process is critical to the successful design and operation of a full-scale commercial system. Credit: Gretchen Ertl
This research was supported by the MIT Department of Mechanical Engineering. Further information can be found in:
Zhang, V.L. Muldoon, and S. Deng. “Accelerated synthesis of Li(Ni0.8Co0.1Mn0.1)O2 cathode materials using flame-assisted spray pyrolysis and additives.” Journal of Power Sources, March 2, 2022. Online: doi.org/10.1016/j.jpowsour.2022.231244.
This article appears in the Winter 2023 issue of Energy Futures.
Press inquiries: miteimedia@mit.edu