
Overview
MIT researchers have developed a process for capturing carbon dioxide (CO2) from power plant exhaust that solves several problems now inhibiting the widespread adoption of conventional “scrubber” technologies. Operating a scrubber uses about a third of the low-pressure steam in a power plant, reducing the output of electricity and significantly increasing its price. Instead of steam, the new system uses electricity to trigger chemical reactions that control the CO2 capture process. Bench-scale experiments and simulation studies show that the new system should capture CO2 as well as scrubbers do but consume less of the plant’s electricity and require far lower capital investment. And because the system doesn’t require major restructuring of steam delivery systems, it could easily be added to existing power plants. Next up: testing at larger scale and under optimized operating conditions.
Power plants using fossil fuels spew out huge quantities of CO2, the greenhouse gas considered most responsible for climate change. Researchers have known how to capture CO2 in power plant exhaust since the 1930s. Flow a plant’s flue gas through a solution containing molecules called amines, and the amines will pluck out the CO2 and hold onto it. The exhaust gases that reach the atmosphere are then cleansed of CO2.

T. Alan Hatton, the Ralph Landau Professor of Chemical Engineering Practice. Photo: Stu Rosner
The challenge is to get the amines to release the CO2 so that the pure CO2 can be either sequestered or utilized and the amines can be recycled and reused. Heating the CO2-amine “complex” up in a separate chamber works, but getting enough steam to both separate the CO2 and sweep it away requires diverting much of the power plant’s steam from its usual job of turning turbines. Additional energy is needed to compress the captured CO2 prior to its utilization or injection into an underground reservoir for long-term storage. Given the reduction in electricity output and the increase in price, it’s not surprising that such thermal scrubbers have not been widely deployed.
At MIT, a team led by T. Alan Hatton, the Ralph Landau Professor of Chemical Engineering Practice, is developing a process that should prove more attractive. Called Electrochemically Mediated Amine Regeneration, or EMAR, the process uses electricity to produce chemical changes that separate the CO2 from the amines. Rather than heating up the entire solvent solution, the approach targets just the CO2-amine molecules that need to be separated; and it doesn’t involve diverting any of the power plant’s steam.
Using electrochemistry to do the CO2–amine separation isn’t a new idea. Research groups have been working on it since the 1970s. But they haven’t been able to come up with an efficient process that uses a widely available, low-cost sorbent and generates a pure CO2 outlet stream. The problem, says Michael C. Stern PhD ’13, now an associate at Exponent, is that they’ve been searching for a single compound that both picks up and releases the CO2 efficiently. The trick was to introduce a second compound. “We decided that the best way to convince the amine to let go of the CO2 would be to give it a better alternative—something that it’d preferentially choose to bind with over the CO2,” says Stern, who initially proposed the idea in 2010 when he was a graduate student in Hatton’s lab. “Then it’ll let go of the CO2 in order to grab this other molecule.”
Chemical reactions in the EMAR process
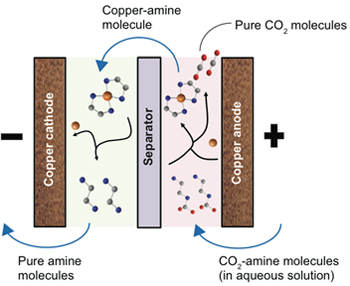
The figure to the right shows the chemistry that occurs inside the electrochemical cell at the heart of the EMAR process. The cell consists of two chambers with copper walls—a positively charged anode in one and a negatively charged cathode in the other. Carried by an aqueous solution, the CO2-amine molecules enter the first chamber, where they come into contact with copper ions released by the copper anode when it’s electrically charged. The amines react with the copper ions, dropping the CO2 molecules in the process. The amines—now carrying tightly bound copper ions—flow into the other chamber, where the copper cathode pulls away the copper ions. The amine molecules are once again unburdened and ready for more CO2. Meanwhile, the other molecules in the solution are unaffected by the electrochemical activity.
Putting the process into practice
The schematic diagram below shows the overall setup of the EMAR system. The electrochemical cell is at the right, an absorber at the left. The CO2-containing flue gas flows upward in the absorber, passing through the amine solution injected near the top. The amines grab out the CO2 molecules, and the remaining flue gas flows out the top. The CO2-amine solution drops to the bottom of the absorber and is pumped to the anode side of the cell. There, the amines pick up copper ions and release CO2 gas into the solution. The gas-liquid mixture goes to the flash tank, where the CO2 exits—no steam required. The solution flows to the cathode side, where the copper-amine molecules lose their copper ions. The empty amine molecules flow back to the absorber to capture more CO2.
Schematic diagram of the EMAR process
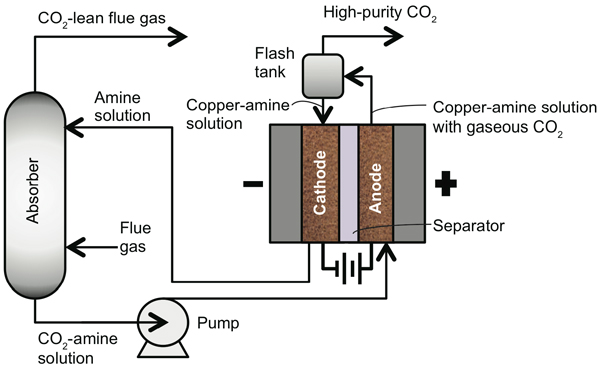
Throughout this process, the copper anode loses material, and the copper cathode gains it. “We can’t let that go on indefinitely or the anode would dissolve away,” says Hatton. “So we solve the problem by swapping sides.” They switch the direction of the current—so the anode becomes the cathode and vice versa—and reverse the direction of the flow through the cell. “That then builds up the side that’s been depleted and depletes the side that’s been built up,” says Hatton. The switching is easily accomplished and could be done automatically.
Like a conventional thermal scrubber, the EMAR technology should remove 90% of the CO2 in flue gas. But it offers significant practical advantages over a scrubber. For one thing, it’s easy to retrofit. Installing a steam system in an existing facility requires major structural changes to redirect tremendous amounts of steam. In contrast, EMAR is a “drop-in technology” that requires only electricity. Also, because the EMAR process doesn’t rely on steam from the operating plant, it’s highly flexible. When electricity supply is strained or prices are high, the flow of exhaust through the capture system can be cut off, and all the plant’s output can be sent to the grid.
In addition, the EMAR system can be used in steam-free environments. For instance, it can be installed in cement, steel, and aluminum manufacturing plants, which together account for nearly 10% of global carbon emissions. And because of its targeted nature, it can be used in settings where CO2 is present in low concentrations but must be removed to protect human health, for example, in submarines, space shuttles, and other confined spaces.
Binding of amines with CO2 and copper
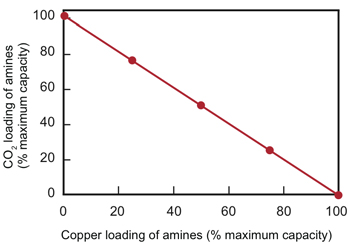
This figure shows the impact of adding copper ions to a CO2-amine solution. When no copper is present, the amines are fully loaded with CO2; they are holding all the CO2 they can. As the copper concentration increases, copper loading of the amines increases, and CO2 loading decreases. Ultimately, the amines are 100% loaded with copper, and all the CO2 has been released into the solution.
Effect of current on CO2 capture
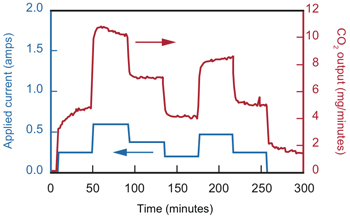
This diagram shows the effect of variations in current within the prototype electrochemical cell on the rate at which CO2 leaves the absorber. As the input current goes up and down over time, the amount of CO2 in the output stream follows the same trend. This result demonstrates that current controls the level of CO2 capture in the system.
Demonstrations in the lab
To demonstrate and test the EMAR process, Stern and Hatton built a bench-scale prototype including all the components in the schematic diagram. Tests in the prototype confirm the effectiveness of the system.
For example, one experiment tested the impact of adding copper ions to the CO2-amine solution. At the start of the experiment, the amines were completely “loaded” with CO2, that is, holding as much as they could, just as they would in the absorber. The researchers then slowly added copper ions, at each step measuring the fraction of the amines combined with CO2 and the fraction combined with copper ions. As shown in the top figure to the right, as the copper-amine fraction increases, the CO2-amine fraction decreases. When the copper loading reaches 100%, the CO2 loading reaches zero. The process has successfully separated all of the CO2 from the amines.
In another series of tests, the researchers measured CO2 output as they varied the input current—a change that affects the rate at which the copper ions are released in the anode and picked up in the cathode. The bottom figure to the right shows the experimental results. Over a period of five hours, the team altered the current every 45 minutes and simultaneously measured the mass of CO2 leaving the cell. The correlation between the input current and CO2 output confirms the effectiveness as well as the flexibility of the EMAR process.
Theoretical simulations, promising outcomes
The bench-scale prototype is, of course, a proof-of-concept system, not yet optimized in terms of design and operating conditions. “Our mathematical models show that the energy consumption of our prototype is far from the lower limit that should be possible with this technology,” says Aly Eltayeb, a graduate student in chemical engineering who is now working on the project.
Right now, the bench-scale system—operating at room temperature and pressure—uses about 100 kilojoules (kJ) of energy to capture a mole of CO2. That level is higher than that achieved with today’s thermal scrubbing processes, which require (depending on the installation) roughly 60 kJ per mole of CO2 captured and compressed. However, the theoretical analyses suggest that optimizing the EMAR system can bring its energy requirement (excluding compression) down toward the 15 kJ per mole limit of the technology. At that point, it will require only 25% of a plant’s electrical output. Also, it should be twice as effective as a thermal scrubber at removing CO2 from the amines. As a result, only a third as much amine solution will need to be recirculated, so the absorber—the most capital-intensive unit in a CO2 capture plant—can be smaller. And the pressure of the exiting CO2 stream will be high enough that only half as much energy-consuming compression will be needed. Finally, the EMAR process runs at a lower temperature than a thermal scrubber does, so there’s less thermal degradation of the amine over time.
What about cost? For benchmarks, the researchers turned to the US Department of Energy (DOE). According to DOE estimates, the cost of using current thermal capture systems is about $61 per ton of CO2 captured. (Costs are in 2012 dollars and will vary at different installations.) To make carbon capture economically competitive, DOE has set a target of $40 per ton by 2025, with the cost dropping to $10 per ton over the following 10 years. The MIT researchers’ “back-of-the-envelope” calculations on the EMAR process suggest a cost of $45–$55 per ton of CO2 captured—not far above DOE’s 2025 target. While the EMAR process may not provide a significant cost advantage over a thermal system in new construction, the estimated cost of EMAR combined with the ease of its installation would mean that retrofitting carbon capture on existing power plants and industrial facilities could become a reality.
Next steps
The researchers are now planning to design and build larger-scale systems in which they can prove—and improve—the system’s performance. They’ll operate the equipment at higher temperatures and pressures and over longer running times to confirm that the process is robust and stable, even with the constant reversal of direction. They are also considering other materials. They started with amines and copper because of the proven ability of the former to capture CO2 and the well-understood electrochemistry of the latter. But they are now examining other possible combinations for their two-molecule approach. While optimizing, scaling up, and preparing the EMAR process for deployment will require significant work, the MIT team believes that the technology has the potential to be ready for widespread commercialization within the next decade.
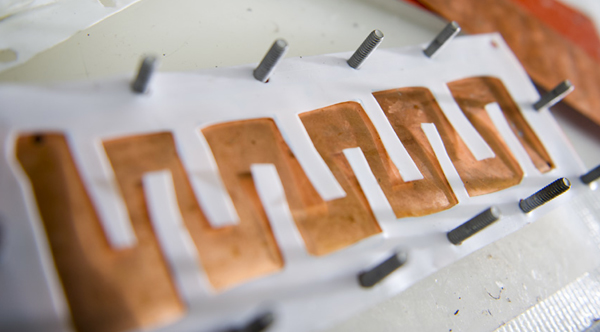
The researchers use this flow channel to guide the amine solution along the copper surfaces inside their electrochemical cell. If the channel were straight, the solution would flow through the cell too quickly. The serpentine shape slows the flow down, increasing the time the solution is in contact with the copper so as to maximize the transfer of copper ions. Photo: Stuart Darsch
This research was supported by Siemens AG and by the US Department of Energy through the Advanced Research Projects Agency–Energy. Further information can be found in:
M.C. Stern. Electrochemically-Mediated Amine Regeneration for Carbon Dioxide Separations. PhD thesis, MIT Department of Chemical Engineering, 2013.
M.C. Stern and T.A. Hatton. “Bench-scale demonstration of CO2 capture with electrochemically-mediated amine regeneration.” RSC Advances, vol. 4, pages 5906–5914, 2014.
M.C. Stern, F. Simeon, H. Herzog, and T.A. Hatton. “Post-combustion carbon dioxide capture using electrochemically mediated amine regeneration.” Energy and Environmental Science, vol. 6, pages 2505–2517, 2013.
This article appears in the Spring 2014 issue of Energy Futures.