Converting carbon dioxide into fuels and chemical feedstocks using renewable sources of electricity may represent a viable strategy for mitigating greenhouse gas emissions responsible for global warming. Fuels synthesized from carbon dioxide would be carbon-neutral once combusted in an engine or oxidized in a fuel cell, while chemical feedstocks may enable long-term sequestration if ultimately used to produce a plastic or resin. At present, selective and efficient routes have been devised for converting carbon dioxide into carbon monoxide and formic acid, but not for alcohol products. Of the various alcohols, the simplest target is the C1 product methanol. Conversion of carbon dioxide into methanol is attractive since it is a liquid which high volumetric energy density which facilitates transport. In addition, there are well-established, efficient routes in the chemical industry for converting methanol to longer-chain hydrocarbons, such as gasoline, as well as commodity chemicals, including olefins, formaldehyde, and formic acid.
Electrochemical carbon dioxide reduction has largely relied upon manipulation of catalysts consisting of single metals to attempt to improve selectivity for a desired product, without tuning the bonding environment surrounding cationic metal centers or the bonding environment of metal nanoparticles. This has hindered the development of electrocatalysts for selectively reducing carbon dioxide at low overpotentials. We will seek to establish how the rich tunability of perovskite oxides, consisting of multiple metal ions in well- ordered arrangements, and nanostructures resulting from their partial reduction, can be used to develop selective catalysts for electrochemical reduction of carbon dioxide to hydrocarbons and alcohols (Figure 1).
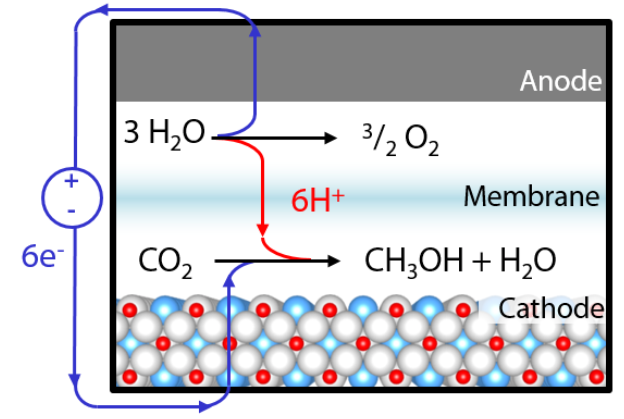
Figure 1. Electrochemical reduction of carbon dioxide to methanol using a perovskite oxide cathode. By powering this process using renewable sources of electricity, it is possible to generate carbon-neutral fuels.
Perovskite oxides are appealing as tunable catalysts because the cationic composition at the A and B sites can be systematically varied without altering the crystal structure, allowing for precise tuning of adsorbate binding energies. Adsorption at both the A- and B-site cations provides additional degrees of freedom for modulating the adsorption energies for both carbon and oxygen bound intermediates, opening up rich possibilities for controlling selectivity in electrochemical carbon dioxide reduction beyond that achieved with single metals. Our key aims include:
- Synthesize faceted nanoparticles of perovskite oxides
- Conduct mechanistic studies to develop a molecular-level picture of carbon dioxide
reduction on perovskite oxides - Integrate perovskite oxides into scalable reactor architectures
Altogether, the proposed efforts will use molecular-level understanding of catalysts to further advancements in practical processes for carbon dioxide reduction.