Listen to the bonus interview:
Katie Luu: From MIT, this is the Energy Initiative. I’m Katie Luu. Today we’re speaking with Betar Gallant, an assistant professor of mechanical engineering at MIT.
Betar Gallant: Hi. I’m very happy to be here.
KL: Thanks for joining us. What do you do here at MIT?
BG: I’m an assistant professor in mechanical engineering. However, my research treats electrochemistry. Different types of electrochemical devices, ranging from lithium ion batteries. We work a bit on developing advanced materials that can go inside the battery, cathode and anode materials, so that we can develop systems with higher energy density for transportation applications.
We also apply electrochemistry to try to tackle environmental challenges. Recently, we’ve been working a lot on designing new electric chemical processes to take CO2 from a gas phase, for example, as emitted from transportation or from a power plant, capture it, and find some valuable and use for the emissions and try to sequester and get it out of the airstream.
KL: What kind of end uses do you envision for the solid CO2?
BG: That’s a great question. This is a really big debate that has been going on for a long time. We have technologies that allow us to think about capturing CO2, essentially filtering it out from a combustion stream that might contain other gases and water and so on. We have those technologies in the world of CO2 capture. They’re being piloted as we speak.
Then the question is, let’s imagine we can safely and effectively capture the CO2. Typically, at that point, we’re talking about getting it into a liquid phase. Some kind of sorbent that will absorb the CO2 selectively. Then the question is, what do we do with it? This is where, depending on what topic you do research in, there are different opinions out there. Right now, essentially, the best we can do or the closest we are to a commercialized process is to essentially heat up that liquid and regenerate the CO2 back to the gas phase. Now we have purified CO2 that’s been separated from a waste stream.
Then what do you do with it? Essentially, the idea is you can pump an underground, so find a reservoir where you can storage and some kind of geological formation, hopefully safely for thousands and thousands of years. That’s known as carbon sequestration. Then there are those of us in the electrochemistry community, we’re very good at, or we like to think we’re good at, figuring out how to shuttle electrons around to drive chemical processes. That’s what we do. There are those of us in the community who are interested in saying, well, there might be some risks associated with underground storage. Also, wouldn’t it be nice if we could make some kind of useful, valuable even product out of the CO2?
The electric chemists try to step in downstream of that capture and regeneration process and say, “Okay, we’ll take the CO2, and we’ll figure out what to make with it.“ But it’s not so easy, because at that point, doing electrochemistry with CO2 is also very challenging. We have a challenging capture and regeneration process. We can talk more about the challenges there. Then we’ve got a challenging electrochemistry process. It turns out what to make is not an easy question to answer.
KL: How much energy goes into current processes for turning CO2 into solids? How does your approach differ?
BG: We can think about the CO2 management process, let’s call it two steps in a series. You have the capture and regeneration step. This would be essentially a system integrated, for example, into a power plant. You can exothermically, or essentially spontaneously, absorb this year too. That just means that the liquid sorbent that we use in these applications really likes to bind with CO2. That’s a so-called energetically free process or downhill process that just spontaneously occurs. That’s great. But then you only have so many sites or molecules in this orbit that can bind with CO2. Eventually, if becomes saturated. Then the liquid is no longer useful for reacting with CO2. At that point, you have to do a process on this liquid to what’s called regenerate the sorbent. Typically, that involves routing the CO2 rich liquid through another part of the system where you heat it up. You can either go to higher temperatures or you can also go to lower pressures. But typically, we think in terms of a higher temperature process. You heat it up and you essentially undo the reaction that happened in the previous step and essentially release the CO2 back to the gas phase.
The issue is that requires quite a lot of energy. You have to heat up not only the specific bonds that you want to break, but typically all of this is occurring in a water-based solution. You have to heat up all of the water as well and that doesn’t really go into driving the reaction that we want, the heating up the water doesn’t necessarily aid with the CO2 liberation, it’s just the direct heating of the sorbent. It turns out that heating up the entire solution just to break a few specific bonds is really energetically inefficient. A typical metric that we use is, a prototypical or a prototype capture system might require something like 30% of the power plant’s rated capacity in order to clean up and regenerate the CO2. That’s a really big problem, 30% we lose upstream just to create a separated purified stream of CO2.
Then there’s a second step, if you’re an electric chemist, because then you take that CO2 and you want to do further reactions with it. Most typically, in the community, folks have been trying to develop what we’ll call aqueous water-based CO2 reactions. The idea is to essentially do what we call a reduction reaction. Basically, you react CO2 with electrons, but you also react it with protons that are available in water. You use the water as a reactant. The idea is to take CO2 from a harmful species and convert it into something useful, for example, methane, which would be potentially usable as a fuel. There’s the value added component. The issue is that you have to put a lot of energy into the system to do an aqueous-based, a water-based CO2 electrochemical reaction.
Herein lies the big challenge. Not only is it intrinsically somewhat energy inefficient to get the products that we want, but now you have to combine that with the upstream energy penalties that we’ve paid to separate and purify this year, too, so the two compound.
KL: What are you doing differently with your research into this?
BG: There’s a lot of really great research going on in the CO2 community. Particularly looking at trying to catalyze the reaction that I just mentioned, the water-based CO2 conversion to a fuel or potentially to another chemical, for example, methanol or formic acid would be useful products to make as well. There’s really a lot of excellent science going on looking at trying to lower some of the energy barriers to try to make the products we want with better selectivity, and in an efficient way. The issue, though, is that there are a lot of challenges with getting both selectivity and high energy efficiency.
When my group was looking at this challenge, we thought, “Okay, this is a very active field. But there are really a lot of hurdles. Maybe we can take a step back and challenge some of the assumptions here.” Namely, the assumption that we need to be making a fuel or a chemical at the end and thus that we need to be doing these reactions in a water-based environment to begin with. We took a step back and we said, “Are there other interesting ways to think about unlocking the reactivity of CO2, so that we can direct it into some other kind of a useful, potentially useful product?” What we thought about was actually to go back to the technology I mentioned earlier, which is CO2 capture. We realized, of course, that there’s this community that understands very well how to get CO2 into a liquid environment, in an exothermic or downhill way, as I mentioned before.
There’s a particular chemical motif, it’s called an amine. Amines are widely used in CO2 capture, typically diluted in water, because they react with CO2 so well and so selectively. When they do that, they also electronically rearrange the CO2. The CO2 gets incorporated effectively into a new species in the liquid phase. We thought, what if we take that motif, which works really well, and we think about now taking this CO2 rich capture solution and instead of regenerating CO2 back to a gas, what if we use that solution as a starting point for further electrochemistry? This is different from what folks in the CO2 defuels community are doing. Because, remember, they’ve already had an upstream capture and regeneration, then they start with the purified CO2, and then they take it to a fuel.
What we thought about is, “What if we can get rid of this intermediate step? We can take CO2 to the captured state in the liquid and then directly use that as an electrolyte, in some kind of an electric chemical system. Can we just directly discharge that solution of that electrolyte? Essentially what do we make?” This was the question that we asked.
There are a lot of reasons why scientifically, it raises a lot of interesting questions too, because we would essentially be reacting CO2, not as CO2, but as this new state in the new molecule that it’s reacted with.
We set about trying to do this reaction. The first step was to really see if it was viable. Meaning, if we try to discharge the CO2 rich liquid, do we see any activity? Do we see any evidence that anything can react? That was one of the first questions that we needed to figure out. What we did a little bit differently is we looked at conducting this reaction in a water-based environment, in an aqueous electrolyte, similar to what the community is generally doing right now. For various reasons, the science was actually pretty messy, because water can actually participate in the reaction and so on. What we did differently was to say, let us isolate the amine in the CO2 by working in a what we call a non-aqueous or a non-water containing electrolyte. We shifted the reaction space from a water-based reaction to something that actually looks a lot more like the environment that you’d find inside of your lithium ion battery.
KL: Can you tell us a bit about how your battery works?
BG: Absolutely. What happens in the lab is we build what is essentially a lithium-based battery. Lithium is the anode material and then we have a carbon cathode. The carbon is the surface on which the CO2 conversion reaction happens. It’s basically there to make sure electrons can get around to the surface of the electrode in a favorable way. But it’s not acting as a catalyst. That’s a key point. Our system doesn’t require any metals to catalyze the reaction. Then in between the two electrodes, you have a non-aqueous or organic electrolyte that contains lithium ions. In the laboratory, we assemble a cell like this and then we can bubble CO2 or purge CO2 through the cell’s head space and create a CO2 rich environment which simulates what, for example, a system in a power plant might be exposed to. Then CO2 gets incorporated into the cell. It also very readily dissolves into the electrolyte because the electrolyte really likes to react with CO2.
Now we have CO2 available in fairly high concentration in our electrolyte. Then we hook it up to our electric chemical equipment. We essentially just directly discharge the battery. In doing so, what we’re doing is we’re reacting the CO2 in the liquid state with the lithium ions that are available, and electrons, and we’re doing what’s called a reduction reaction. Essentially what we learned is, during that process, we can undo the bond that originally formed between the amine and the CO2. We can essentially nicely selectively pluck off the CO2 from the electrolyte molecule and react just the CO2 and not the amine.
This is the key point, just the CO2 to another product. Essentially, what the special electrolyte is doing is, it’s acting as a medium to have a very high concentration of CO2 around and it also activates the CO2 and makes it a lot easier to cycle it to some type of a product.
The question is, what product do we make? Because we’ve got CO2 around and we’ve got a lot of lithium around, indeed, what we make is a lithium carbonate species. We discharge the battery and we make from initially a liquid, and even before that a gas phase CO2, in the end, we make a solid phase, which is a lithium carbonate solid that grows inside the electrode as we discharge it.
Then we have two options as to what we do with that lithium carbonate. The first option is we can, potentially. down the road devise a process where we remove it from the cell. The reason that’s potentially attractive is we’ve now taken CO2 and in an energetically favorable way to a solid phase. A solid phase is really easy to handle. You can remove it from the system and clean it up a little bit, but essentially, pretty readily dispose of it. Now CO2 is in a solid form. It’s not compressed underground, stored underground where there’s a potential risk of release. Now it’s essentially sequestered for good in a solid. That’s one of the potential end uses. In that case, you would really think about just discharging the battery potentially even once. That starts to look like what we would describe as a primary battery.
Another thing you can do is decompose the lithium carbonate electric chemically. Once you incorporate the CO2 into the battery, you can seal it off, discharge it, make lithium carbonate. Then using electrochemistry, you could decompose the lithium carbonate and it would go back to CO2 and liberate the lithium ions that were consumed in the process. That CO2 would then stay within the closed system, so within the battery. It turns out, you can actually cycle the system like this, between lithium carbonate and then back to CO2, for a number of cycles. Now, it doesn’t cycle extremely well. This is not a battery that one would potentially drop into replace lithium ion batteries and their Tesla anytime soon. But it definitely does cycle and with some more research may one day get to a point where it can really function well as a rechargeable system. There are a lot of hurdles that need to be figured out or jumped over before that really looks viable. But that’s another potential and end use of the system.
KL: What made you pick lithium and not another material?
BG: I’m so glad you asked. Lithium is really not a good choice. Lithium is expensive. Even more importantly, it’s not Earth abundant. We essentially long term need to be working with materials that are sustainable. We have a great interest in moving beyond lithium, potentially looking at sodium, for example. This is something that we’re working on now.
The reason we started with lithium is that we know as scientists and as electrochemists, we’re very comfortable integrating electrolytes with lithium and cathode materials with lithium because we can take a page from the battery community. I mentioned earlier that my group works a lot on lithium ion and lithium battery technology. We had a lot of lithium around. But we also have the know-how for how to safely construct an electrochemical system that would essentially let us answer the question that we raised in the beginning, is it even possible, does such a reaction work? Once we could answer that question as a proof of concept, we thought, then we can really start looking into the best material to replace lithium.
KL: Do you think power plants would be more apt to help this technology advance by using a different material? Because lithium is expensive. I’m trying to think how to make this more of a scalable reality.
BG: It’s interesting. It depends who the customer is. Our battery, once packaged up, once there’s CO2 contained in the electrolyte, from the user’s point of view, everything after that, when you discharge the battery, the user gets power out. You can envision a system where the power plant operators are utilizing the system to sequester CO2. On site at least, they’re also getting some power back in the process. That’s in contrast to the case I mentioned in the beginning where you need to pay about 30% of the power plant’s capacity to take care of the CO2. On site that’s potentially a win. But in the grand scheme of things, there are upstream costs. In the end, what we really care about is the overall impact on the planet. There are upstream energetic costs and also economic costs that had to go into acquiring the lithium and also making the electrolyte in the first place.
The question is, who really pays for that, and in the end, can the gains that we think our technology may deliver with more work, can they really offset these upstream costs? That requires drawing a bigger box around the problem, which we’re doing right now. We’re doing the technoeconomic analysis and the lifecycle analysis to try to really pinpoint the answer to your question earlier, what do you do with the CO2? We have shown scientifically that this is a really viable pathway. But now we need to figure out, do the economics work out? Ultimately, is it really a win for the planet? We hope so.
KL: Out of all the hurdles you’ve mentioned in this whole process, which do you think is the biggest?
BG: I could give you a list of hurdles that we haven’t even talked about yet. It’s been really fun working on this system because we identified a new class of reactions involving CO2. It’s not that easy to do. Chemists and engineers and material scientists and electrochemists have been working on and studying CO2 for decades and decades. We stumbled into this really interesting new series of reactions. We’re at a point where we’re just understanding all of the variables that really affect how the reaction proceeds and what products you get out and how that affects the performance of the battery.
We’ve opened up this really great scientific landscape. That’s really exciting. It also means that we are still too early on to really have the metrics to be able to say, here’s what the file system design is going to look like, whether it’s better used as an open or a closed system, whether you continuously have CO2 flowing through it or whether it’s something where you flow a fixed amount of CO2 and you seal it up and you use it as a rechargeable battery. We’re still figuring those things out. That’s occurring in parallel with the science. Both are going to inform what the best end use for this technology is going to be. It’s very exciting. We need more people to work on this to help us answer that question.
KL: How long have you been working on this problem to get to where you are right now?
BG: We started working on the CO2 project about when I started at MIT. That was in 2016. My very first PhD student started to tackle this project and I think she didn’t know what she was getting into. I think I didn’t either, and that’s probably to the benefit of all of us because it turned out to be really challenging. Once you know as simple as a battery or an electric chemical cell, and you see that something works, so you’re getting some current out of it, that’s great. But then you have to really dig into it and figure out and identify what reaction is going on. Because electrons can really go any number of places in a new environment, and you have to really do the science to figure out that they’re going to the place you wanted them to go. It was a long endeavor to really test our first hypothesis, that we think that this can work, but also to prove that we understood the science inside the cell. That was a multi-year effort.
We published our first paper on that in 2018. Since then, it’s opened all kinds of doors. We’re moving ahead with more fundamentals of the science right now. It’s going to be a long term project. Eventually, in the end, I’m a mechanical engineer. What we want to be doing are designing at the systems level as well.
KL: What is one thing that you hope listeners will take away from hearing about your research?
BG: I think it’s good to take risks and try to think outside of the box. Try to find the spaces where you perceive people are not looking. If you are willing to explore those areas, then you can find delightful things, and also interesting work to do. I think we need, more and more, we need students, researchers, people who are willing to have one foot in one community or one research topic and another foot in another and say, hey, I’ve identified some interesting and unique overlap here and I think it’s worth exploring.
KL: Where can we keep up with your research?
BG: You can go to my website where we publish our papers as they’re coming out, gallant.mit.edu. That’s going to have the latest research findings.
KL: Thank you so much for coming to chat with us. It was such a pleasure.
BG: Thanks. It was great to be here.
In brief
Some power plants use materials called sorbents to remove carbon dioxide (CO2) from their exhaust so it can be sequestered from the environment. But separating the CO2 from the sorbent requires high temperatures and produces CO2 gas that must be put into long-term storage—a prospect that raises safety and security concerns. In a proof-of-concept study, MIT researchers have demonstrated a battery-like system that uses the same CO2-capturing sorbent in a specially designed electrolyte that drives electrochemical reactions with three benefits: They separate the CO2 from the sorbent; they promote the discharge of electricity from the battery; and they incorporate the CO2 into a solid that can serve as electrode material or be safely discarded. Their system—made of lithium and carbon electrodes plus the special electrolyte—achieves discharge voltages similar to those of other lithium-gas batteries under development. The researchers are now working to understand and optimize their lithium-based system and to see whether less-expensive, earth-abundant metals might work as well.
Reducing CO2 emissions from power plants is widely considered an essential component of any climate change mitigation plan. Many research efforts focus on developing and deploying carbon capture and sequestration (CCS) systems to keep CO2 emissions from power plants out of the atmosphere. But separating the captured CO2 and converting it back into a gas that can be stored can consume up to 25% of a plant’s power-generating capacity. In addition, the CO2 gas is generally injected into underground geological formations for long-term storage—a disposal method whose safety and reliability remain unproven.
A better approach would be to convert the captured CO2 into useful products such as value-added fuels or chemicals. To that end, attention has focused on electrochemical processes—in this case, a process in which chemical reactions release electrical energy, as in the discharge of a battery. The ideal medium in which to conduct electrochemical conversion of CO2 would appear to be water. Water can provide the protons (positively charged particles) needed to make fuels such as methane. But running such “aqueous” (water-based) systems requires large energy inputs, and only a small fraction of the products formed are typically those of interest.
Betar Gallant, an assistant professor of mechanical engineering, and her group have therefore been focusing on non-aqueous (water-free) electrochemical reactions—in particular, those that occur inside lithium-CO2 batteries.
Research into lithium-CO2 batteries is in its very early stages, according to Gallant, but interest in them is growing because CO2 is used up in the chemical reactions that occur on one of the electrodes as the battery is being discharged. However, CO2 isn’t very reactive. Researchers have tried to speed things up by using different electrolytes and electrode materials. Despite such efforts, the need to use expensive metal catalysts to elicit electrochemical activity has persisted.
Given the lack of progress, Gallant wanted to try something different. “We were interested in trying to bring a new chemistry to bear on the problem,” she says. And enlisting the help of the sorbent molecules that so effectively capture CO2 in CCS seemed like a promising way to go.
Rethinking amine
The sorbent molecule used in CCS is an amine, a derivative of ammonia. In CCS, exhaust is bubbled through an amine-containing solution, and the amine chemically binds the CO2, removing it from the exhaust gases. The CO2—now in liquid form—is then separated from the amine and converted back to a gas for disposal.
In CCS, those last steps require high temperatures, which are attained using some of the electrical output of the power plant. Gallant wondered whether her team could instead use electrochemical reactions to separate the CO2 from the amine—and then continue the reaction to make a solid, CO2-containing product. If so, the disposal process would be simpler than it is for gaseous CO2. The CO2 would be more densely packed, so it would take up less space; and it couldn’t escape, so it would be safer. Better still, additional electrical energy could be extracted from the device as it discharges and forms the solid material. “The vision was to put a battery-like device into the power plant waste stream to sequester the captured CO2 in a stable solid, while harvesting the energy released in the process,” says Gallant.
Research on CCS technology has generated a good understanding of the carbon-capture process that takes place inside a CCS system. When CO2 is added to an amine solution, molecules of the two species spontaneously combine to form an “adduct,” a new chemical species in which the original molecules remain largely intact. In this case, the adduct forms when a carbon atom in a CO2 molecule chemically bonds with a nitrogen atom in an amine molecule. As they combine, the CO2 molecule is reconfigured: It changes from its original, highly stable, linear form to a “bent” shape with a negative charge—a highly reactive form that’s ready for further reaction.
In her scheme, Gallant proposed using electrochemistry to break apart the CO2-amine adduct—right at the carbon-nitrogen bond. Cleaving the adduct at that bond would separate the two pieces: the amine in its original, unreacted state, ready to capture more CO2, and the bent, chemically reactive form of CO2, which might then react with the electrons and positively charged lithium ions that flow during battery discharge (see the diagram below). The outcome of that reaction could be the formation of lithium carbonate (Li2CO3), which would deposit on the carbon electrode.
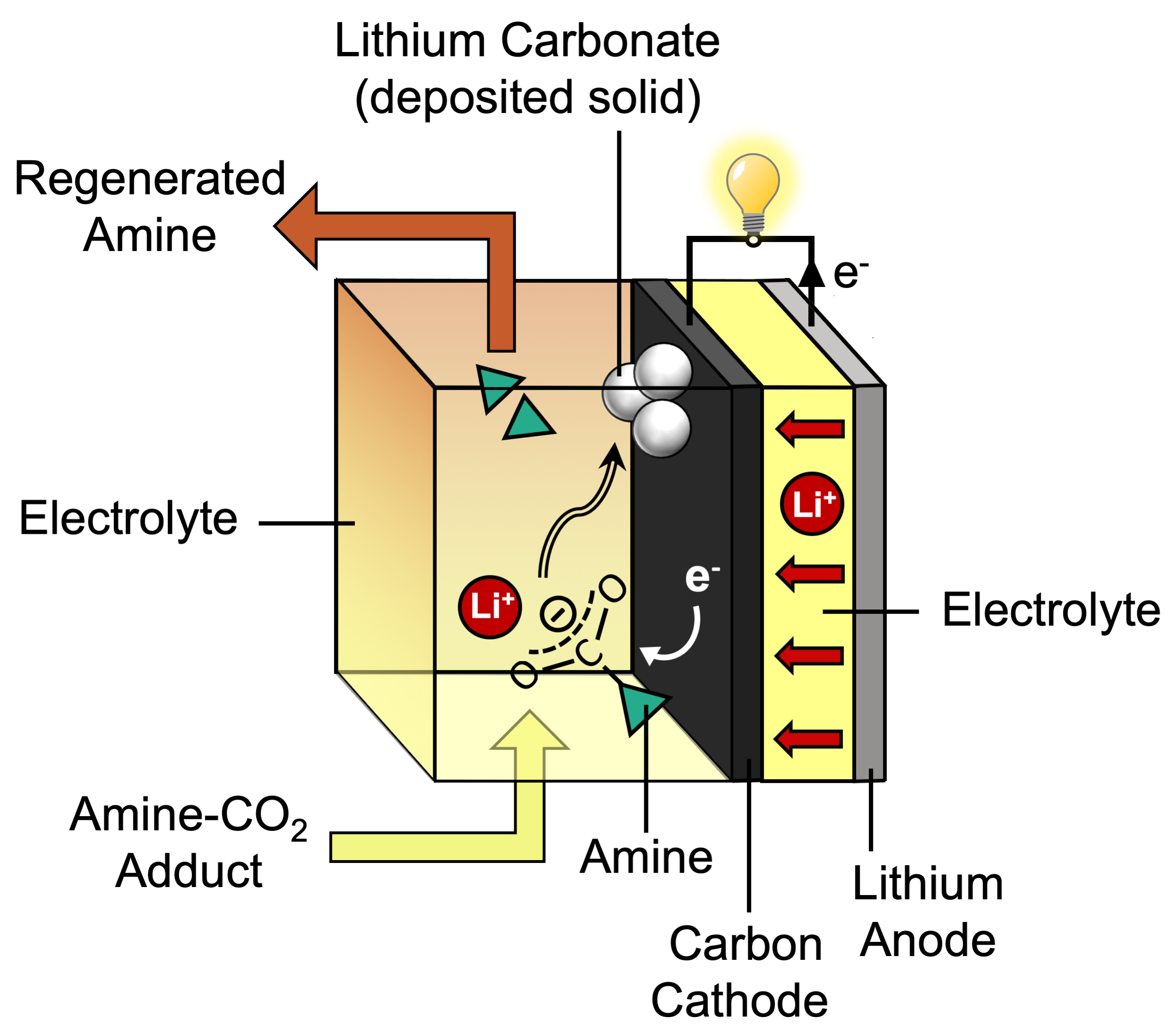
At the same time, the reactions on the carbon electrode should promote the flow of electrons during battery discharge— even without a metal catalyst. “The discharge of the battery would occur spontaneously,” Gallant says. “And we’d break the adduct in a way that allows us to renew our CO2 absorber while taking CO2 to a stable, solid form.”
A process of discovery
In 2016, Gallant and doctoral student Aliza Khurram of mechanical engineering began to explore that idea.
Their first challenge was to develop a novel electrolyte. A lithium-CO2 battery consists of two electrodes—an anode made of lithium and a cathode made of carbon—and an electrolyte, a solution that helps carry charged particles back and forth between the electrodes as the battery is charged and discharged. For their system, they needed an electrolyte made of amine plus captured CO2 dissolved in a solvent—and it needed to promote chemical reactions on the carbon cathode as the battery discharged.
They started by testing possible solvents. They mixed their CO2-absorbing amine with a series of solvents frequently used in batteries and then bubbled CO2 through the resulting solution to see if CO2 could be dissolved at high concentrations in this unconventional chemical environment. None of the amine-solvent solutions exhibited observable changes when the CO2 was introduced, suggesting that they might all be viable solvent candidates.
However, for any electrochemical device to work, the electrolyte must be spiked with a salt to provide positively charged ions. Because it’s a lithium battery, the researchers started by adding a lithium-based salt—and the experimental results changed dramatically. With most of the solvent candidates, adding the salt instantly caused the mixture either to form solid precipitates or to become highly viscous—outcomes that ruled them out as viable solvents. The sole exception was the solvent dimethyl sulfoxide, or DMSO. Even when the lithium salt was present, the DMSO could dissolve the amine and CO2.
“We found that—fortuitously—the lithium-based salt was important in enabling the reaction to proceed,” says Gallant. “There’s something about the positively charged lithium ion that chemically coordinates with the amine-CO2 adduct, and together those species make the electrochemically reactive species.”
Exploring battery behavior during discharge
To examine the discharge behavior of their system, the researchers set up an electrochemical cell consisting of a lithium anode, a carbon cathode, and their special electrolyte—for simplicity, already loaded with CO2. They then tracked discharge behavior at the carbon cathode.
As they had hoped, their special electrolyte actually promoted discharge reaction in the test cell. “With the amine incorporated into the DMSO-based electrolyte along with the lithium salt and the CO2, we see very high capacities and significant discharge voltages—almost 3 volts,” says Gallant. Based on those results, they concluded that their system functions as a lithium-CO2 battery with capacities and discharge voltages competitive with those of state-of-the-art lithium-gas batteries.
The next step was to confirm that the reactions were indeed separating the amine from the CO2 and further continuing the reaction to make CO2-derived products. To find out, the researchers used a variety of tools to examine the products that formed on the carbon cathode.
In one test, they produced images of the post-reaction cathode surface using a scanning electron microscope (SEM). Immediately evident were spherical formations with a characteristic size of 500 nanometers, regularly distributed on the surface of the cathode (see the image below). According to Gallant, the observed spherical structure of the discharge product was similar to the shape of Li2CO3 observed in other lithium-based batteries. Those spheres were not evident in SEM images of the “pristine” carbon cathode taken before the reactions occurred (see the image below).
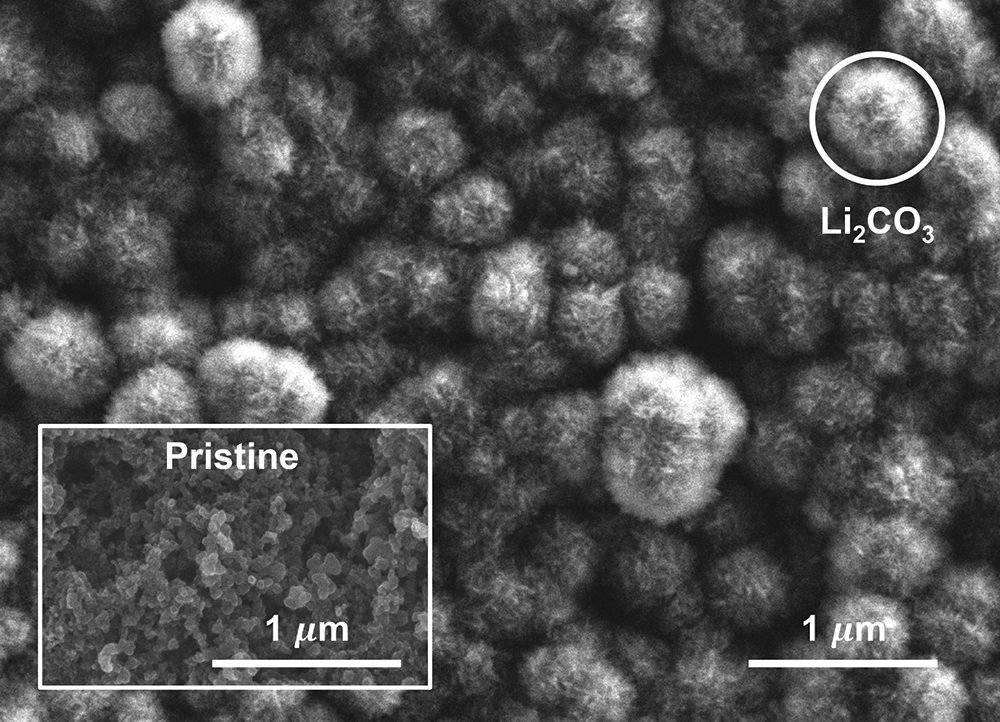
Other analyses confirmed that the solid deposited on the cathode was Li2CO3. It included only CO2-derived materials; no amine molecules or products derived from them were present. Taken together, those data provide strong evidence that the electrochemical reduction of the CO2-loaded amine occurs through the selective cleavage of the carbon-nitrogen bond.
“The amine can be thought of as effectively switching on the reactivity of the CO2,” says Gallant. “That’s exciting because the amine commonly used in CO2 capture can then perform two critical functions. It can serve as the absorber, spontaneously retrieving CO2 from combustion gases and incorporating it into the electrolyte solution. And it can activate the CO2 for further reactions that wouldn’t be possible if the amine were not there.”
Future directions
Gallant stresses that the work to date represents just a proof-of-concept study. “There’s a lot of fundamental science still to understand,” she says, before the researchers can optimize their system.
She and her team are continuing to investigate the chemical reactions that take place in the electrolyte as well as the chemical makeup of the adduct that forms—the “reactant state” on which the subsequent electrochemistry is performed. They are also examining the detailed role of the salt composition.
In addition, there are practical concerns to consider as they think about device design. One persistent problem is that the solid deposit quickly clogs up the carbon cathode, so further chemical reactions can’t occur. In one configuration they’re investigating—a rechargeable battery design—the cathode is uncovered during each discharge-charge cycle. Reactions during discharge deposit the solid Li2CO3, and reactions during charging lift it off, putting the lithium ions and CO2 back into the electrolyte, ready to react and generate more electricity. However, the captured CO2 is then back in its original gaseous form in the electrolyte. Sealing the battery would lock that CO2 inside, away from the atmosphere—but only so much CO2 can be stored in a given battery, so the overall impact of using batteries to capture CO2 emissions would be limited in this scenario.
The second configuration the researchers are investigating—a discharge-only setup—addresses that problem by never allowing the gaseous CO2 to re-form. “We’re mechanical engineers, so what we’re really keen on doing is developing an industrial process where you can somehow mechanically or chemically harvest the solid as it forms,” Gallant says. “Imagine if by mechanical vibration you could gently remove the solid from the cathode, keeping it clear for sustained reaction.” Placed within an exhaust stream, such a system could continuously remove CO2 emissions, generating electricity and perhaps producing valuable solid materials at the same time.
Gallant and her team are now working on both configurations of their system. “We don’t know which is better for applications yet,” she says. While she believes that practical lithium-CO2 batteries are still years away, she’s excited by the early results, which suggest that developing novel electrolytes to pre-activate CO2 could lead to alternative CO2 reaction pathways. And she and her group are already working on some.
One goal is to replace the lithium with a metal that’s less costly and more earth-abundant, such as sodium or calcium. With seed funding from the MIT Energy Initiative, the team has already begun looking at a system based on calcium, a material that’s not yet well-developed for battery applications. If the calcium-CO2 setup works as they predict, the solid that forms would be calcium carbonate—a type of rock now widely used in the construction industry.
In the meantime, Gallant and her colleagues are pleased that they have found what appears to be a new class of reactions for capturing and sequestering CO2. “CO2 conversion has been widely studied over many decades,” she says, “so we’re excited to think we may have found something that’s different and provides us with a new window for exploring this topic.”
This research was supported by startup funding from the MIT Department of Mechanical Engineering. Mingfu He, a postdoc in mechanical engineering, also contributed to the research. Work on a calcium-based battery is being supported by the MIT Energy Initiative Seed Fund Program. Further information can be found in:
A. Khurram, M. He, and B.M. Gallant. “Tailoring the discharge reaction in Li-CO2 batteries through incorporation of CO2 capture chemistry.” Joule, vol. 2, pp. 1–18, December 19, 2018. Online: doi.org/10.1016/j.joule.2018.09.002.
This article appears in the Spring 2019 issue of Energy Futures.