
Imagine charging up the battery in your electric vehicle in five minutes, then accelerating rapidly to join traffic on the highway. That’s the vision of MIT scientists who are looking to speed up the flow of electricity into and out of rechargeable batteries. Their recent research findings provide atomic-level insights into what limits that flow — and why fabricating electrodes from nanoscale particles eases the problem.
Yet-Ming Chiang of materials science and engineering, the Kyocera Professor of Ceramics, is no stranger to powerful rechargeable batteries. In past research, he and his colleagues developed an intrinsically safe, long-lasting lithium-ion battery that was five times more powerful than its competitors. The battery, marketed since 2001 by A123 Systems of Watertown, has been incorporated into high-powered cordless tools and is now being demonstrated in plug-in hybrid electric vehicles.
Despite their success, Chiang and his colleagues — like others in the field — have been uncertain as to why high power is so hard to achieve. While many experts cite limited rates of chemical diffusion, Chiang suggested instead that the impediment is how quickly structural changes can occur at the atomic level — a hypothesis that’s been supported by results from recent experimental and theoretical work.
When a lithium-ion battery produces electricity, lithium ions flow from the negative electrode to the positive electrode of the battery. The faster those ions move, the higher the battery’s power. The limiting factor has been how quickly the positive electrode can take up the lithium ions. And how quickly that electrode can release lithium ions has limited the rate at which the battery can be recharged.
Among the electrode materials that work best are olivines, a family of crystalline compounds with a similar atomic-scale structure. For a given olivine, the structure differs slightly depending on whether lithium is present or not. As a result, when lithium ions move into or out of the positive electrode, a new crystalline structure must form in place of the original one. “So the challenge is to engineer, or design, the material to allow that structural or phase change to happen as quickly as possible because the faster it can go, the higher the power that the battery can deliver and the faster it can be recharged,” Chiang said.
But in a conventional electrode, there is a problem: the change from one crystalline structure to the other gets stuck. Consider the flow of lithium into the positive electrode as a charged-up battery is discharged to run a device. “We start off with a positive electrode that contains no lithium,” Chiang explained. “As we add lithium, part of that solid modifies its crystalline structure. Now there’s a mismatch between that new structure and the original structure in the part of the solid that’s still devoid of lithium.” The mismatch at the interface between the two crystalline structures slows the uptake of lithium ions, which limits the power of the battery.
The key, then, is to prevent the material from separating into regions with and without lithium. But stable crystalline structures exist for only two compositions: when the material is completely free of lithium and when it’s fully saturated with lithium. No stable crystalline structure exists for intermediate concentrations of lithium, such as those present when the battery is discharging or charging. As a result, the material quickly separates into lithium-saturated and lithium-free regions.
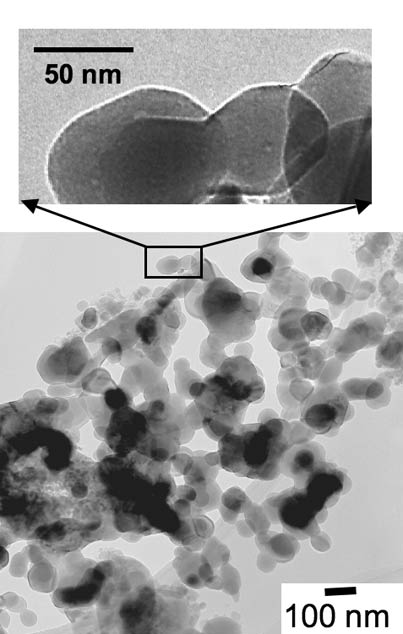
These images show the nanoscale particles that make up the electrodes of a doped lithium iron phosphate battery — an intrinsically safe rechargeable battery developed at MIT and remarkable for its high power, long lifetime, and mechanical robustness. Recent MIT research has provided atomic-level insights into why working at the nanoscale makes those characteristics possible.
The nanotech advantage
In conventional forms of these compounds, the only way to achieve stability at intermediate lithium concentrations is by raising the temperature — not a practical solution for a battery electrode. Another approach is to use nanotechnology. “The fascination with nanomaterials is that unusual things happen when particles get very small,” said Chiang. “As the size scale gets smaller and smaller, physical properties such as melting points and optical and electronic properties can change dramatically.”
Indeed, when the researchers tested electrode materials made up of particles less than 100 nm in diameter, they observed regions with intermediate lithium concentrations — even at room temperature. In the overall material, the composition was more mixed, reducing or eliminating abrupt interfaces between regions of differing composition. And when that condition prevailed, the battery being tested had much higher power. “Therefore we reasoned that these two are coupled phenomena,” Chiang said. “By reducing the particle size to the nanoscale we allow the transition from lithium-devoid to lithium-saturated and vice versa to proceed much faster.”
Moreover, in tests with different olivines the researchers found that some exhibited the desired behavior far more than others. That finding could explain why certain olivines exhibit exceptional performance as electrode materials while others do not — a discrepancy that has been puzzling to battery experts. A good criterion for identifying promising new electrode compounds may therefore be their ability to adsorb and release lithium without abrupt and significant structural change. As an added benefit, the use of such compounds should extend battery lifetime. Eliminating abrupt changes in composition will reduce cracking and other damage that can occur with repeated charging and discharging.
Initially, Chiang was reluctant to use the term “nanotechnology” when talking about his ongoing work on battery technology. However, the new fundamental results have convinced him that he and his team are indeed using nanotechnology to engineer and control the physical properties of their electrode materials. “There have previously been observations of [this type of] change in behavior at the nanoscale,” he said. “But this is the first time that we’re aware of that it’s been observed in a battery-storage material and actually used as a mechanism for achieving higher performance.”
This research was sponsored by the United States Advanced Battery Consortium and a Royal Thai Government Graduate Fellowship. For more information, see Advanced Functional Materials, v. 17, 2007, pp. 1115-1123.
This article appears in the Winter 2008 issue of Energy Futures.