
As the world looks for ways to stop climate change, much discussion focuses on using hydrogen instead of fossil fuels, which emit climate-warming greenhouse gases (GHGs) when they’re burned. The idea is appealing. Burning hydrogen doesn’t emit GHGs to the atmosphere, and hydrogen is well-suited for a variety of uses, notably as a replacement for natural gas in industrial processes, power generation, and home heating.
But while burning hydrogen won’t emit GHGs, any hydrogen that’s leaked from pipelines or storage or fueling facilities can indirectly cause climate change by affecting other compounds that are GHGs, including tropospheric ozone and methane, with methane impacts being the dominant effect. A much-cited 2022 modeling study analyzing hydrogen’s effects on chemical compounds in the atmosphere concluded that these climate impacts could be considerable. With funding from the MIT Energy Initiative’s Future Energy Systems Center, a team of MIT researchers took a more detailed look at the specific chemistry that poses the risks of using hydrogen as a fuel if it leaks.
The researchers developed a model that tracks many more chemical reactions that may be affected by hydrogen and includes interactions among chemicals. Their results showed that while the impact of leaked hydrogen on the climate wouldn’t be as large as the 2022 study predicted—and that it would be about a third of the impact of any natural gas that escapes today—leaked hydrogen will impact the climate. Leak prevention should therefore be a top priority as the hydrogen infrastructure is built, state the researchers.
Hydrogen’s impact on the “detergent” that cleans our atmosphere
Global three-dimensional climate-chemistry models using a large number of chemical reactions have also been used to evaluate hydrogen’s potential climate impacts, but results vary from one model to another, motivating the MIT study to analyze the chemistry. Most studies of the climate effects of using hydrogen consider only the GHGs that are emitted during the production of the hydrogen fuel. Different approaches may make “blue hydrogen” or “green hydrogen,” a label that relates to the GHGs emitted. Regardless of the process used to make the hydrogen, the fuel itself can threaten the climate. For widespread use, hydrogen will need to be transported, distributed, and stored—in short, there will be many opportunities for leakage. The question is, What happens to that leaked hydrogen when it reaches the atmosphere? The 2022 study predicting large climate impacts from leaked hydrogen was based on reactions between pairs of just four chemical compounds in the atmosphere. The results showed that the hydrogen would deplete a chemical species that atmospheric chemists call the “detergent of the atmosphere,” explains Candice Chen, a PhD candidate in MIT’s Department of Earth, Atmospheric and Planetary Sciences (EAPS). “It goes around zapping greenhouse gases, pollutants, all sorts of bad things in the atmosphere. So it’s cleaning our air.” Best of all, that detergent—the hydroxyl radical, abbreviated as OH—removes methane, which is an extremely potent GHG in the atmosphere. OH thus plays an important role in slowing the rate at which global temperatures rise. But any hydrogen leaked to the atmosphere would reduce the amount of OH available to clean up methane, so the concentration of methane would increase.
However, chemical reactions among compounds in the atmosphere are notoriously complicated. While the 2022 study used a “four-equation model,” Chen and her colleagues—Susan Solomon, the Lee and Geraldine Martin Professor of Environmental Studies and Chemistry; and Kane Stone, a research scientist in EAPS—developed a model that includes 66 chemical reactions. Analyses using their 66-equation model showed that the four-equation system didn’t capture a critical feedback involving OH—a feedback that acts to protect the methane-removal process.
Here’s how that feedback works. As the hydrogen decreases the concentration of OH, the cleanup of methane slows down, so the methane concentration increases. However, that methane undergoes chemical reactions that can produce new OH radicals. “So the methane that’s being produced can make more of the OH detergent,” says Chen. “There’s a small countering effect. Indirectly, the methane helps produce the thing that’s getting rid of it.” And, says Chen, that’s a key difference between their 66-equation model and the four-equation one. “The simple model uses a constant value for the production of OH, so it misses that key OH-production feedback,” she says.
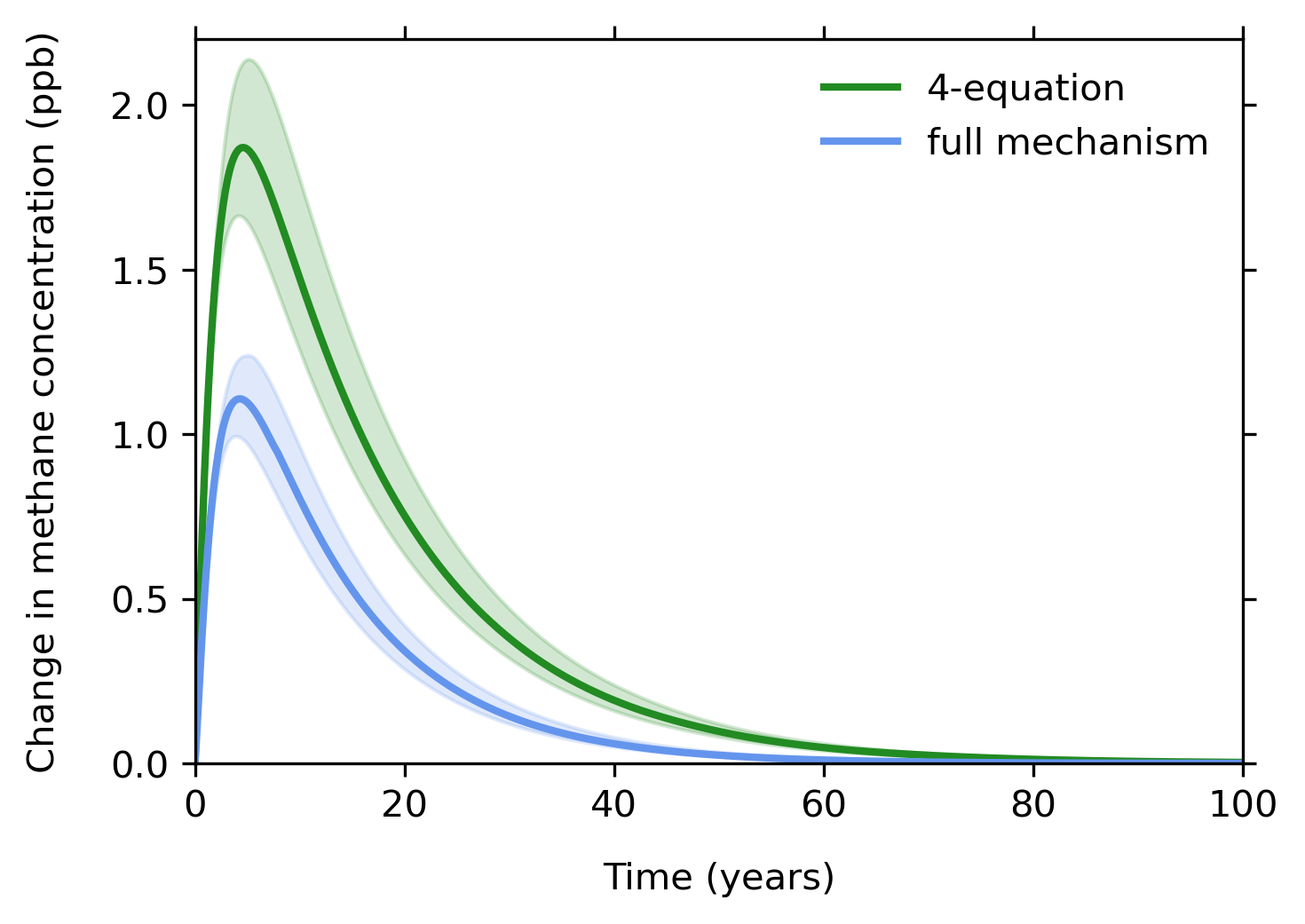
Calculating the impact of hydrogen on atmospheric methane: Sample results using the two models
The figure above demonstrates the importance of that feedback effect. The figure plots the change in methane concentration (the vertical axis) versus time in years (the horizontal axis). The MIT researchers assume that a single pulse of hydrogen is injected at time zero and then use the two models to calculate the additional methane concentration (measured in parts per billion, or ppb) over the next 100 years. Results from the four-equation model are shown in green, while those from the 66-equation model appear in blue. In each case, the shaded areas represent uncertainty due to the model parameters used, such as temperature, pressure, strength of hydrogen soil sink, and more. With the four-equation system, the additional methane concentration peaks at nearly 2 ppb; with the 66-equation system, it peaks at just over 1 ppb.
Because the four-equation analysis assumes only that the injected hydrogen destroys the OH, the methane concentration increases unchecked for the first 10 years or so. In contrast, the 66-equation analysis goes one step further: The methane concentration does increase, but as the system re-equilibrates, more OH forms and removes methane. By not accounting for that feedback, the four-equation analysis overestimates the peak increase in methane due to the hydrogen pulse by about 85%. Spread over time, the simple model doubles the amount of methane that forms in response to the hydrogen pulse.
Chen cautions that the point of their work is not to present their result as “a solid estimate” of the impact of hydrogen. Their analysis is based on a simple “box” model that represents global average conditions and assumes that all the chemical species present are well mixed. Thus, the species can vary over time—that is, they can be formed and destroyed—but any species that are present are always perfectly mixed. As a result, a box model does not account for the impact of, say, wind on the distribution of species. “The point we’re trying to make is that you can go too simple,” says Chen. “If you’re going simpler than what we’re representing, you will get further from the right answer.” She goes on to note, “The utility of a relatively simple model like ours is that all of the knobs and levers are very clear. That means you can explore the system and see what affects a value of interest.”
Leaked hydrogen versus leaked natural gas: A climate comparison
Burning natural gas produces fewer GHG emissions than does burning coal or oil; but as with hydrogen, any natural gas that’s leaked from wells, pipelines, and processing facilities can have climate impacts, negating some of the perceived benefits of using natural gas in place of other fossil fuels. After all, natural gas consists largely of methane, that highly potent GHG in the atmosphere that’s cleaned up by the OH detergent. Given its potency, even small leaks of methane can have a large climate impact.
So when thinking about replacing natural gas fuel—essentially methane—with hydrogen fuel, it’s important to consider how the climate impacts of the two fuels compare if and when they’re leaked. The usual way to compare the climate impacts of two chemicals is using a measure called the global warming potential, or GWP. The GWP combines two measures: the radiative forcing of a gas—that is, its heat-trapping ability—with its lifetime in the atmosphere. Since the lifetimes of gases differ widely, to compare the climate impacts of two gases, the convention is to relate the GWP of each one to the GWP of carbon dioxide.
But hydrogen and methane leakage cause increases in methane, and that methane decays according to its lifetime. Chen and her colleagues therefore realized that an unconventional procedure would work: They could compare the impacts of the two leaked gases directly. What they found was that the climate impact of hydrogen is about three times less than that of methane (on a per mass basis). So switching from natural gas to hydrogen would not only eliminate combustion emissions but also potentially reduce the climate effects, depending on how much leaks.
Key take-aways
In summary, Chen highlights some of what she views as the key findings of the study. First on her list is the following: “We show that a really simple four-equation system is not what should be used to project out the atmospheric response to more hydrogen leakages in the future.” The researchers believe that their 66-equation model is a good compromise for the number of chemical reactions to include. It generates estimates for the GWP of methane “pretty much in line with the lower end of the numbers that most other groups are getting using much more sophisticated climate chemistry models,” says Chen. And it’s sufficiently transparent to use in exploring various options for protecting the climate. Indeed, the MIT researchers plan to use their model to examine scenarios that involve replacing other fossil fuels with hydrogen to estimate the climate benefits of making the switch in the coming decades.
The study also demonstrates a valuable new way to compare the greenhouse effects of two gases. As long as their effects exist on similar time scales, a direct comparison is possible—and preferable to comparing each with carbon dioxide, which is extremely long-lived in the atmosphere. In this work, the direct comparison generates a simple look at the relative climate impacts of leaked hydrogen and leaked methane—valuable information to take into account when considering switching from natural gas to hydrogen.
Finally, the researchers offer practical guidance for infrastructure development and use for both hydrogen and natural gas. Their analyses determine that hydrogen fuel itself has a “non-negligible” GWP, as does natural gas, which is mostly methane. Therefore, minimizing leakage of both fuels will be necessary to achieve net-zero carbon emissions by 2050, the goal set by both the European Commission and the U.S. Department of State. Their paper concludes, “If used nearly leak-free, hydrogen is an excellent option. Otherwise, hydrogen should only be a temporary step in the energy transition, or it must be used in tandem with carbon-removal steps [elsewhere] to counter its warming effects.”