
Overview
In the race to develop the perfect energy storage solution, ultracapacitors are an exciting horse to bet on. They deliver energy quickly, can be recharged in seconds, and have a long life span—but their capacity for storing energy is limited. An MIT startup company has now unveiled a novel version that can store twice as much energy and deliver about 10 times as much power as a conventional device can. Equipped with carbon-nanotube-coated electrodes, the new ultracapacitor uses low-cost, domestically abundant materials and a manufacturing process similar to those used at large scale by the solar industry. Among the first likely technologies enabled by the new ultracapacitor: a new hybrid electric vehicle that combines fuel efficiency with high performance and dramatically lower cost.
In the growing effort to run cars on electricity and generate power from solar and wind resources, a major stumbling block is energy storage. One promising energy-storage technology is the ultracapacitor, a device that offers significant advantages over the best of today’s batteries. For example, ultracapacitors can provide high power—that is, they can deliver energy quickly; they can be recharged in seconds rather than hours; they can withstand cold temperatures, shocks, and vibrations; and they can be charged and discharged hundreds of thousands of times before they wear out. They also contain earth-abundant and nontoxic materials, so they are much easier on the environment than today’s batteries are.
Ultracapacitors do, however, have one serious drawback: their low energy-storage capacity. At an equivalent size, an ultracapacitor can store only about 5% as much energy as a lithium ion battery can. Today, millions of ultracapacitors are used in battery-powered consumer products, providing backup power or brief bursts of energy in microcomputers, cellphones, and cameras. But an ultracapacitor capable of high energy storage could transform the energy scene, making possible high-performance, energy-efficient hybrid and electric vehicles, smoothly operating solar- and wind-powered grids, and more.
A question of storing ions
The key to energy storage—whether in a battery or an ultracapacitor—is the ability to transfer and store charged particles called ions, says Joel Schindall, the Bernard Gordon Professor of the Practice in MIT’s Department of Electrical Engineering and Computer Science. Both devices have at their core an electrolyte, a mixture of positive and negative ions. In a battery, chemical reactions move ions from the electrolyte into and out of the atomic structure of the electrode material as the battery is charged and discharged. In contrast, in an ultracapacitor, an electric field causes the ions to move to and from the surfaces of the electrodes. Because the ions just cling on and then let go—with no chemical reaction involved—an ultracapacitor can charge and discharge quickly, again and again. But while the battery stores ions throughout its electrodes—where there are many spaces for them to reside until the battery is discharged—the ultracapacitor stores them only on its surfaces.
In theory, then, the solution to ultracapacitor energy storage is simple: provide more electrode surface area for ions to cling onto. In today’s commercial ultracapacitors, electrode surfaces are coated with activated charcoal, a material that is full of pores, providing surface area for clinging ions. But energy storage is still low.
In 2004, Schindall proposed a different solution: coat the electrodes instead with vertically aligned carbon nanotubes. A tightly packed array of tall, thin nanotubes on the electrode could provide lots of surface area for the clinging ions. Also, while the pores in activated carbon are irregular in size and shape, a nanotube “forest” would provide straight pathways so the ions could come in and out easily and pack together neatly—like sucking up paint with a paintbrush rather than a sponge, says Schindall. He began to explore the concept with collaborators John G. Kassakian, professor of electrical engineering, and Riccardo Signorelli, then a graduate student in electrical engineering and computer science and subsequently a postdoctoral associate in the Laboratory for Electromagnetic and Electronic Systems (now part of MIT’s Research Laboratory of Electronics).
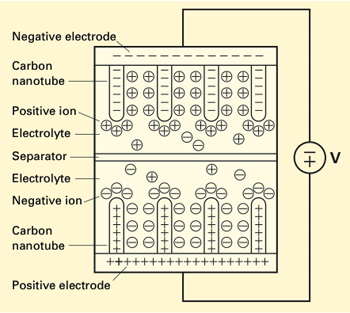
Schematic diagram of the nanotube-enhanced ultracapacitor. At the top and bottom of the device are electrode plates with vertically oriented carbon nanotubes. (The nanotubes are relatively much longer and denser than indicated in this schematic.) Because of the applied voltage, the electrodes are coated with ions of the opposite charge and act as capacitors to produce voltage and store charge. They remain that way until the external circuit is connected. Electrons then flow from the negative to the positive electrode, powering a device along the way. The electrodes gradually lose their charge, and the ions let go and once again distribute themselves throughout the electrolyte.
The concept and first steps
This diagram shows the researchers’ “nanotube-enhanced ultracapacitor.” At the top and bottom are the two electrode plates with carbon nanotubes attached vertically. A liquid electrolyte fills the space between them, and a porous separator down the middle keeps the plates from electrically shorting together. In this diagram, a voltage across the two plates has induced an excess of negative charge (electrons) on the top plate and an excess of positive charge (the absence of electrons) on the bottom one. As a result, the nanotubes are coated by ions of the opposite charge. When the two plates are connected by an external loop of wire, electrons will flow through that external circuit from the negative to the positive electrode, powering an electricity-consuming device along the way. Over time, both plates will lose their charge, and the positive and negative ions will break away and mix back into the electrolyte.
With funding from the Ford-MIT Alliance, the MIT team performed detailed simulation studies that confirmed the potential benefits of the proposed concept. The simulations showed that the nanotube-enhanced ultracapacitor should be able to store more ions than conventional activated-carbon ones can, thereby achieving higher energy storage.
Encouraged by those findings, Schindall and Signorelli proceeded to the next challenge: making nanotube-enhanced electrodes. Within a year, they had learned to grow carbon nanotubes on silicon—but silicon is not a good conductor. Growing nanotubes on a conducting surface proved to be more difficult. After testing many materials, designs, and methods, they found a combination that worked. They used a layer of tungsten, then a thin layer of aluminum—the conductor—and finally a top layer of iron oxide, the catalyst for the process. Using the specially designed furnace, they heated up their sample, and the iron oxide separated into droplets. They then blew dilute acetylene gas across the surface. The droplets of iron oxide grabbed carbon out of the gas, and carbon nanotubes began to grow upward out of the droplets. “Each droplet served as a follicle—almost like a hair follicle—for the nanotube growth,” says Schindall. Experiments showed that starting with a very thin layer of iron oxide led to the formation of tiny droplets and the growth of nanotubes that were tall, thin, and closely packed—a configuration that maximizes available surface area on the electrode.

This cross section shows a “forest” of carbon nanotubes grown on an aluminum electrode. Each nanotube is about 250 microns long—about twice as long as a human hair is thick. The densely packed nanotubes significantly increase the surface area of the electrode—the key to the enhanced energy-storage capacity of the new ultracapacitor. Image: Riccardo Signorelli PhD ’09, MIT
The ultimate test: making a device
The next step was to integrate their nanotube-enhanced electrodes into a device and test its function. “We had grown nanotubes of about the right dimensions on a conducting substrate, but we didn’t know how they would work electrically,” says Schindall. He had a list of possible “showstoppers” that could crop up when they tried to assemble a device. For example, could they get the electrolyte to go down between the nanotubes and coat their surfaces? Carbon nanotubes are known to be highly water repellent. In addition, in this application, adjacent nanotubes hold the same charge, and their tips are close together. Would ions be able to pass through the electric field created by those charged tips? And would the nanotubes be able to pick up charge from the base? After all, they are grown on iron oxide, which is an insulator, not a conductor. Answer any of those questions “no,” and the nanotube-enhanced ultracapacitor was not destined for success.
With funding from an MIT Energy Initiative seed grant, the researchers were able to fabricate a prototype test cell that allayed those concerns. They started with their nanotube-coated electrodes in a vacuum and then let air push the electrolyte down past the nanotube tips to fill the space. The ions were able to access and coat all the nanotube surfaces, and the nanotubes were electrically connected. Further studies showed that the base of each nanotube extended beyond the iron oxide droplet from which it had grown. Ultimately, its “foot” surrounded and encompassed the droplet; as a result, it was directly connected to the aluminum substrate below. The prototype thus proved the practical viability of the nanotube-enhanced ultracapacitor.
Getting it to market
The MIT work showed that the new ultracapacitor could store energy, but the demonstration devices were each the size of a thumbnail and could charge and discharge only tiny amounts of energy. Nevertheless, Signorelli believed that they had potential. “Transforming that proof of concept into a full-scale, high-performance, marketable device would require much more development work—but we were confident we could make it happen,” he says.
During the past four years, Signorelli and his colleagues have done just that. In 2008, Signorelli PhD ’09 and John Cooley PhD ’11 founded FastCAP Systems, a company aimed at commercializing the nanotube-enhanced capacitor along with systems to enable its practical implementation. In fall 2009, FastCAP won a $5.3 million award in the first round of the US Department of Energy (DOE) Advanced Research Projects Agency-Energy (ARPA-E) grants—one of just 37 successful proposals out of 3,600 initial submissions. Funding from other sources followed, and in fall 2011, the company received a second DOE grant for deploying the ultracapacitor in the energy market. FastCAP is now housed in a 17,000-square-foot R&D and pilot production facility in the Seaport District of Boston. It has 25 employees and recently sold and shipped its first generation of products.
The latest FastCAP ultracapacitor stores twice as much energy as its competitors can and delivers 7 to 15 times more power. It also costs less. It uses raw materials that are both inexpensive and abundant within the United States. (The electrode material, for instance, costs about one-fiftieth as much as that used in conventional capacitors.) The manufacturing process is based on methods used for large-scale production of solar photovoltaic components. As a result, it is both low-cost and scalable—and as a bonus, the necessary equipment and expertise are highly developed and readily available.
While the new ultracapacitor has potential applications in many fields, the immediate focus is on transportation. Signorelli cites significant opportunities for improving vehicle technology. For example, in an electric car, high-energy-density batteries can provide enough energy to travel 200 miles before recharging. But adding nanotube-enhanced ultracapacitors to such systems would provide high power for acceleration and deceleration and would allow the batteries to be optimized for range rather than for power.
In a hybrid-electric vehicle, the ultracapacitor could be the best option, providing power for rapid acceleration and deceleration and instant discharging and charging—a million or more times over the lifetime of the vehicle. “Most people don’t associate the word ‘hybrid’ with a high-performance vehicle, but our ultracapacitors could change that,” says Signorelli. “Integrating them into today’s hybrid technology could yield new hybrids that are fuel efficient, high performance, and cost competitive with non-hybrid vehicles on the market today.”

Carbon-nanotube-coated chips made at MIT ultimately led to the development of enhanced ultracapacitors for large-scale energy storage. Now, MIT researchers are looking into using them at a tiny scale, embedded within integrated circuits for power switching. Photo: Stuart Darsch
Funding for the MIT research came initially in part from the Ford-MIT Alliance and subsequently from a seed grant from the MIT Energy Initiative. In 2009-2010, John Cooley received support as a Martin Family Fellow for Sustainability. FastCAP has received two grants from the US Department of Energy—one from the Advanced Research Projects Agency and the other from the Office of Energy Efficiency and Renewable Energy—with additional funding through the Chesonis Family Foundation and the Massachusetts Clean Energy Center. Further information can be found on the FastCAP website at www.fastcapsystems.com and in the following publications:
J. Schindall. “What’s in a name? A new model for regenerative electrical energy storage.” IEEE Power Electronics Society Newsletter, first quarter 2008, pages 32–34.
J. Schindall. “The charge of the ultracapacitors.” IEEE Spectrum, November 2007.
R. Signorelli. High Energy and Power Density Nanotube-Enhanced Ultracapacitor Design, Modeling, Testing, and Predicted Performance. PhD thesis, MIT Department of Electrical Engineering and Computer Science, June 2009.
This article appears in the Spring 2012 issue of Energy Futures.