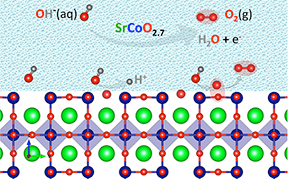
Mobilizing oxygen atoms from the crystal surface of perovskite-oxide electrodes to participate in the formation of oxygen gas is key to speeding up water-splitting reactions, researchers at MIT, the Skoltech Institute of Technology, and the University of Texas at Austin show in a new paper published online in Nature Communications. This work is crucial for the widespread adoption of water splitting to produce hydrogen fuel, an attractive way to depart from traditional energy sources such as fossil fuels towards clean, renewable energy sources.
In the study, Earth-abundant non-precious metal oxide catalysts best known as perovskites were investigated. In particular, the researchers studied perovskite oxide catalysts consisting of varying proportions of lanthanum and strontium with cobalt and oxygen. Experimentalists from the University of Texas at Austin and the Skoltech Center for Electrochemical Energy Storage identified two key parameters governing the catalytic performance of these materials: the covalency of the cobalt-oxygen bond and the number of oxygen vacancies. They then tuned their synthesis parameters to demonstrate that one particular material, strontium cobaltite (SrCoO2.7), exhibits highly active water electrolysis, much faster than the state-of-art catalyst iridium oxide, which contains precious metals. Through collaboration with MIT theorists, the team unraveled how the reaction occurs on the atomic scale. “By using ab initio density functional theory calculations, we illustrated an unprecedented new electrolysis pathway occurring on SrCoO2.7, and showed that it occurs as a result of the nature of the electronic structure in cobalt and oxygen,” MIT mechanical engineering graduate student Xi (Jerry) Rong says. “This shortcut-like pathway with an exceptionally low energy barrier provides essential understanding not only for the development of highly-efficient catalysts, but also for future catalyst optimization.’’
“The generation of oxygen from water remains a significant bottleneck in the development of water electrolyzers and also in the development of fuel cell and metal-air battery technologies. If we could develop catalysts made with Earth-abundant materials that could reversibly and efficiently electrolyze water into hydrogen and oxygen, we could have affordable hydrogen generation from renewables — and with that, the possibility of electric cars that run on water with ranges similar to gas powered cars,” says University of Texas at Austin graduate student and lead author of the study J. Tyler Mefford.
Metal oxide perovskite catalysts in these electrochemical reactions are often viewed as unaffected by the chemical interactions they stimulate, but Rong and MIT Assistant Professor Alexie M. Kolpak show through theoretically based models and simulations that in the most-efficient process — essentially a fast-kinetic shortcut — dehydrogenated hydroxide ions adsorb to the catalyst surface and combine directly with oxygen from the oxide lattice. The consumed lattice oxygen atoms are replaced by hydroxides from the water, thereby returning the catalyst to its initial state to restart the cycle. This mechanism is in contrast to the conventionally assumed reaction pathway, in which only the transition metal lattice sites are involved.
New reaction mechanism
Kolpak and Rong previously provided a theoretical framework of this new mechanism on lanthanum nickel oxide, a well-known efficient perovskite catalyst. This work, published in January in ACS Catalysis, was based on a 2013 study by experimentalists at the University of Texas, led by Keith J. Stevenson, one of the new paper’s co-senior authors and director of the Skoltech Center for Electrochemical Energy Storage. The theory study validated the active role of lattice oxygen atoms in electrolysis, as proposed in the 2013 study, and provided an updated, detailed mechanism, which was enabled by the use of density functional theory modeling. In addition, this work demonstrated a fundamental correlation between electrolysis mechanism and catalyst stability, which may lead to discovery of new catalysts that might otherwise be missed. “When you assume the wrong mechanism, you can make the wrong prediction, which means you then could discard materials that might be really good,” Kolpak suggests.
Their breakthrough illustrates the power of collaborative work to demonstrate the intimate marriage of experimental and theoretical approaches to verify and predict new paradigms for water electrolysis. This work quantitatively explains and consolidates over 40 years of experimental observations, Stevenson says.
Composition-structure-property relationships
A key measurement is overpotential, the amount of energy required to initiate a desirable production rate of molecular oxygen at the electrode surface. “We’ve developed new fundamental relationships between the stability of the bulk oxide, the properties of the surface, and how the actual mechanism of the reaction proceeds, and therefore identified the limitations for improving the efficiency of the water splitting reaction,” Kolpak says. “Now we understand the key structural and compositional parameters for designing new materials based on these fundamental properties. That’s very valuable for guiding the discovery of advanced materials,” she says.
Commenting on the research, Argonne National Laboratory’s principal materials scientist Dillon D. Fong says, “The results are extremely important and timely: While defects have been known to mediate the chemical behavior of oxide surfaces, the authors have done a thorough job of showing exactly how this can occur through a series of highly detailed and systematic studies, both experimental and theoretical.”
“While much work needs to be performed with regard to the overall stability of perovskite catalysts, this communication is unique in that it establishes new guidelines for the rational design of catalysts. Past efforts have focused more on the atomic and electronic structure of ideal perovskites: This work clearly shows the importance of defect engineering for enhancing chemical functionalities,” says Fong, who was not involved in the research.
Stability versus reactivity
Less-stable oxide materials, with their weaker bonds between metal atoms and oxygen atoms, will more easily form oxygen vacancies on the surface, and so should serve as more active catalysts, the new research shows. However, there is a tradeoff between stability and reactivity, because if the material is too unstable, it will break down, perhaps dissolving in the water. “You have to find that balance or find a way to prevent the degradation of the material, without affecting the activity,” Kolpak says.
“Lattice oxygen participation is a prerequisite for the high efficiency of the catalyst, so how to tune the compositions, how to tune the properties, of the catalysts to be beneficial for this mechanism is the key fundamental step for designing catalysts in the future,” Rong says.
Other co-authors of the Nature Communications paper are William G. Hardin of the University of Texas at Austin; Artem M. Abakumov of Skoltech CEES and the University of Antwerp; and Sheng Dai of Oak Ridge National Laboratory.